1) The document presents a summary of a presentation on using oral anticoagulants in patients with acute coronary syndrome.
2) It reviews several clinical trials that evaluated adding direct oral anticoagulants to antiplatelet therapy after ACS and found it reduced ischemic events but increased bleeding risk.
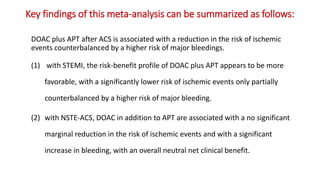
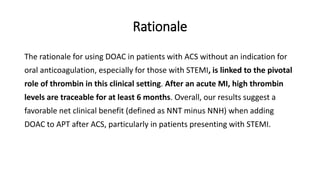
3) A meta-analysis of over 29,000 patients from 6 trials found the benefits of adding a DOAC were greater for those with STEMI compared to NSTEMI, with a lower risk of ischemic events outweighing the higher bleeding risk for STEMI patients.






![Pathophysiology of ACS
• Currently in-hospital treatment of ACS includes both antiplatelet and
anticoagulant medications long-term care focuses primarily on antiplatelet
therapy.
• Despite dual-antiplatelet therapy, patients with stabilized ACS have an ≈9%
to 11% risk of suffering a recurrent adverse cardiovascular event within 1
year.[*]
Platelet adhere to collagen
and vWF
Tissue factor
activation & Factor Xa
Thrombin
activation
Thrombus formation,
expansion, platelet activation
* Wallentin L, Becker RC, Budaj A, et al.; PLATO Investigators. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl JMed. 2009;361:1045–1057.](https://image.slidesharecdn.com/welcometojournalpresentation-181014190008/85/Welcome-to-journal-presentation-7-320.jpg)
![Rationale of anticoagulants use in ACS
• Thrombin levels stay elevated for months after an ACS event[*].
Persistently elevated thrombin levels elevate risk of adverse events despite
antiplatelet therapy. Addition of anticoagulation to the long-term care of
patients with stabilized ACS to lower thrombin levels and improve
outcomes.
• Warfarin, when added to aspirin for long-term therapy after ACS reduced
recurrent MI but Novel oral anticoagulants (NOACs) are more promising
than warfarin.
*Christersson C, Oldgren J, Bylock A, Wallentin L, Siegbahn A. Long-term treatment with ximelagatran, an oral direct thrombin inhibitor, persistently reduces the
coagulation activity after a myocardial infarction. J ThrombHaemost. 2005;3:2245–2253.](https://image.slidesharecdn.com/welcometojournalpresentation-181014190008/85/Welcome-to-journal-presentation-8-320.jpg)
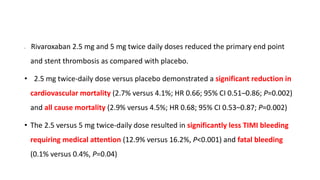
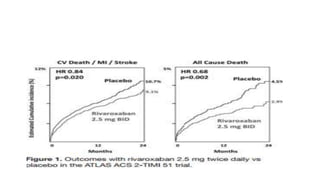





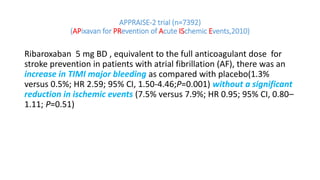

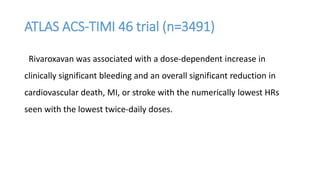


![IMPORTANCE: ACS remain at high risk for experiencing recurrent
ischemic events. Direct oral anticoagulants (DOAC) have been proposed
for secondary prevention after ACS.
OBJECTIVE: safety and efficacy of DOAC in addition to antiplatelet
therapy (APT) after ACS, focusing on treatment effects stratified by
baseline clinical presentation: [NSTE-ACS] vs [STEMI-ACS]](https://image.slidesharecdn.com/welcometojournalpresentation-181014190008/85/Welcome-to-journal-presentation-23-320.jpg)

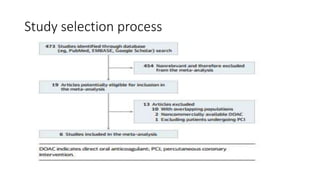

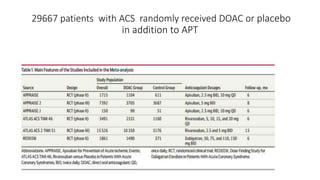
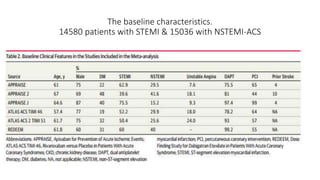
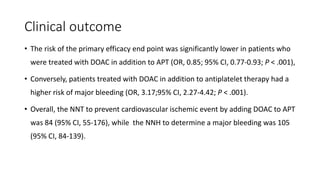
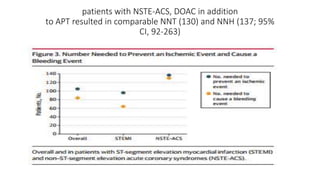
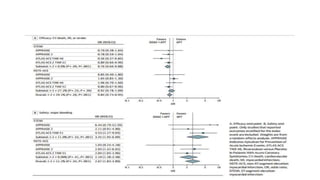
![Results of Six trials that included 29 667 patients (14 580 patients [49.1%]
with STEMI and 15 036 [50.7%] with NSTE-ACS).
• This benefit was pronounced with STEMI (OR, 0.76; 95% CI, 0.66-0.88;
P < .001), while no significant treatment effect with NSTE-ACS (OR,
0.92; 95% CI, 0.78-1.09; P = .36; P for interaction = .09).
• DOACs were associated with a higher risk of major bleeding as
compared with APT alone (OR, 3.17; 95% CI, 2.27-4.42; P < .001)](https://image.slidesharecdn.com/welcometojournalpresentation-181014190008/85/Welcome-to-journal-presentation-31-320.jpg)