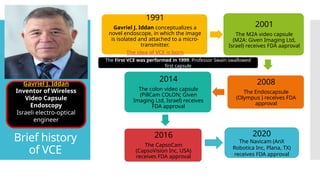
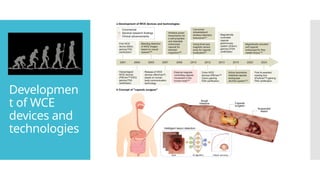
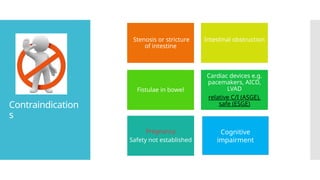
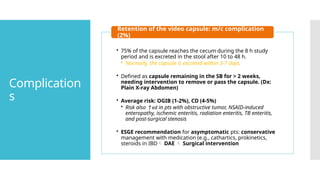
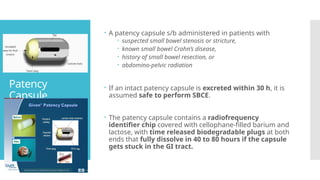
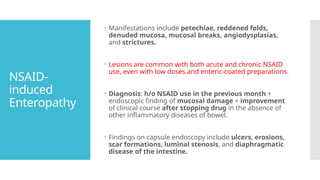
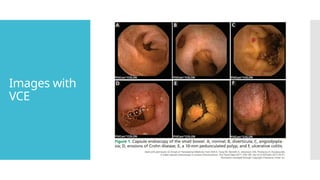
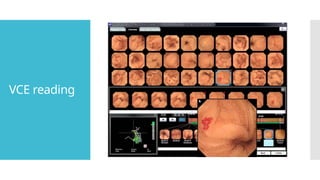
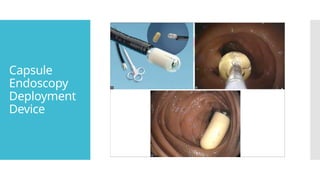
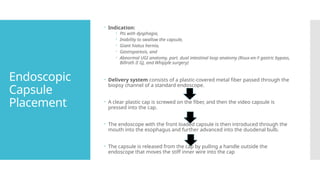
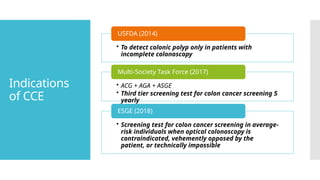
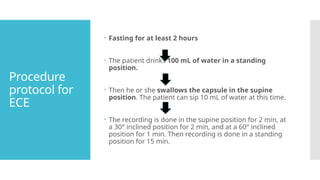
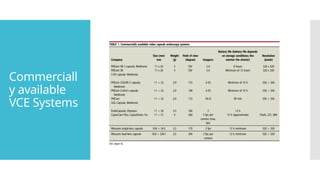
Video capsule endoscopy (VCE) is a non-invasive imaging technique that enables doctors to visualize the small intestine, and in some cases, the esophagus and colon. This method uses a disposable, pill-sized camera that wirelessly transmits images to an external recorder. The primary reasons for performing VCE include investigating obscure gastrointestinal bleeding (OGIB), suspected or confirmed Crohn's disease (CD), small bowel tumors, celiac disease, and polyposis syndrome.



![INTRODUCTIO
N
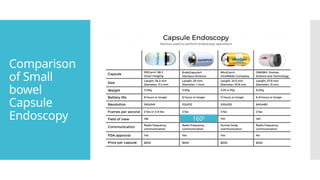
Capsule endoscopes measure 24 to 32 mm in length and 11 to
13 mm in diameter, depending on the manufacturer and product
line.
All capsule endoscopes have similar components: a disposable
plastic-coated capsule + metal oxide semiconductor or high-
resolution charge-coupled device (CCD) image capture system
+ a compact lens + LED illumination sources + internal battery
source.
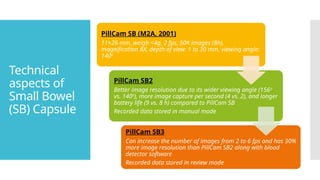
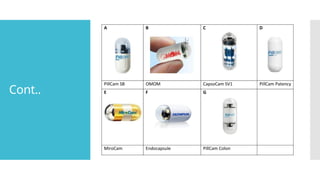
The mode of data transmission from the capsule is either via
ultra-high frequency band radio telemetry (PillCam [Medtronic,
USA], EndoCapsule [USA]) or human body communications
(Mirocam, Intromedic Seoul, Seoul, South Korea).
All available software can identify red pixels to facilitate detection
of bleeding lesions](https://image.slidesharecdn.com/videocapsuleendoscopy-250407163927-5feb72db/85/VIDEO-CAPSULE-ENDOSCOPY-VCE-Introduction-Evolution-Indications-4-320.jpg)

![Basic
components
of Capsule
Endoscopy
1) The capsule: one or more cameras + light source + battery +
transmitter
2) Sensors: placed on the surface of the abdomen or contained in a belt
worn by the patient that is connected to a recorder
3) Software: to process and display images
Devices have improved over the years, with wider field of view (140°–360°),
more cameras (up to 4 in some models), longer battery life, and variable
frame rates: 2 fps when traveling slowly through the stomach and
intestines and up to 35 fps when traveling quickly through the distal
esophagus.
In its journey through the GI tract, the capsule can acquire 50,000 to
60,000 images, which can take from 30 to 90 minutes to review.
Real-time viewing is particularly important in detecting active GI
bleeding.
RAPID Real-Time [Given Imaging/Medtronic,USA], Real Time Viewer [Olympus,
USA], and MiroView Express [IntroMedic, Seoul, South Korea])](https://image.slidesharecdn.com/videocapsuleendoscopy-250407163927-5feb72db/85/VIDEO-CAPSULE-ENDOSCOPY-VCE-Introduction-Evolution-Indications-6-320.jpg)