This study examined the effects of chronic exposure to nicotine on the function of human α4β2 nicotinic acetylcholine receptors (nAChRs), which play a major role in nicotine addiction. The key findings were:
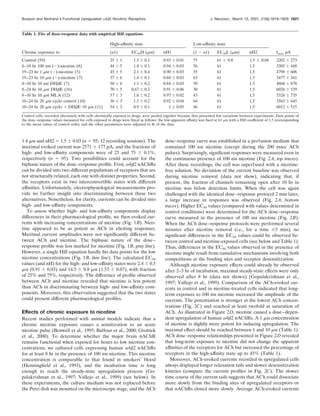
1) Acetylcholine dose-response curves for human α4β2 nAChRs expressed in human cells were biphasic, consisting of both high- and low-affinity components.
2) Chronic exposure to nicotine or nicotinic antagonists increased the fraction of high-affinity receptors from 25% to around 70% and doubled the acetylcholine-evoked currents.
3) Upregulation of receptor function after chronic nicotine exposure occurred
![Chronic Exposure to Nicotine Upregulates the Human ␣42
Nicotinic Acetylcholine Receptor Function
Bruno Buisson and Daniel Bertrand
Department of Physiology, Medical Faculty, 1211 Geneva 4, Switzerland
Widely expressed in the brain, the ␣42 nicotinic acetylcholine
receptor (nAChR) is proposed to play a major role in the mech-
anisms that lead to and maintain nicotine addiction. Using the
patch-clamp technique and pharmacological protocols, we ex-
amined the consequences of long-term exposure to 0.1–10 M
nicotine in K-177 cells expressing the major human brain ␣42
receptor. The acetylcholine dose–response curves are biphasic
and revealed both a high- and a low-affinity component with
apparent EC50 values of 1.6 and 62 M. Ratios of receptors in
the high- and low-affinity components are 25 and 75%, respec-
tively. Chronic exposure to nicotine or nicotinic antagonists
[dihydro--erytroidine (DHE) or methyllycaconitine (MLA)] in-
creases the fraction of high-affinity receptors up to 70%. Up-
regulated acetylcholine-evoked currents increase by twofold or
more and are less sensitive to desensitization. Functional up-
regulation is independent of protein synthesis as shown by the
lack of effect of 20 M cycloheximide. Single-channel currents
recorded with 100 nM acetylcholine show predominantly high
conductances (38.8 and 43.4 pS), whereas additional smaller
conductances (16.7 and 23.5 pS) were observed with 30 M
acetylcholine. In addition, long-term exposure to dihydro--
erytroidine increases up to three times the frequency of channel
openings. These data indicate, in contrast to previous studies,
that human ␣42 nAChRs are functionally upregulated by
chronic nicotine exposure.
Key words: acetylcholine; nicotinic receptor; ␣42; nicotine;
upregulation; nicotine addiction
Nicotine is a tobacco compound that binds specifically to neuro-
nal nicotinic acetylcholine receptors (nAChRs) of the brain (for
review, see Dani and Heinemann, 1996; Changeux et al., 1998)).
Tobacco addiction results from the repetitive intake of nicotine
present in cigarette smoke and its rapid diffusion to the CNS
(Gamberino and Gold, 1999; Leshner and Koob, 1999). Postmor-
tem autoradiographies of smokers’ brains have revealed a higher
density of [H3
]–nicotine binding compared with matched controls
(Perry et al., 1999). Moreover, numerous studies have shown that
long-term exposures to nicotine (or other nAChRs ligands) pro-
duce an increase of the total amount of brain labeling by [3
H]–
nicotine (Marks et al., 1985, 1992; Lapchak et al., 1989; Flores et
al., 1992, 1997; Koylu et al., 1997; Perry et al., 1999). In rodents,
it was proposed that chronic nicotine injection led to the conver-
sion of a fraction of low-affinity nAChRs into high-affinity recep-
tors (Romanelli et al., 1988). However, a consensus derived from
initial observations is that long-term exposure to nicotine causes
an increase in the number of binding sites at the cell surface
(Wonnacott, 1990; Peng et al., 1994). Known as “upregulation,”
this mechanism is opposite to “downregulation,” which was pro-
posed for seven transmembrane receptors such as opiate recep-
tors (Creese and Sibley, 1981).
The reinforcing effects of nicotine implicate 2-containing
nAChRs (Picciotto et al., 1995, 1998) probably by modulating the
release of dopamine in the mesolimbic system (Pidoplichko et al.,
1997). In the striatum, the modulation of dopamine release de-
pends on ␣42 nAChRs (Sharples et al., 2000). Dopamine release
from brain striatal synaptosomes or from striatal slices could be
potentiated (Rowell and Wonnacott, 1990; Yu and Wecker, 1994)
or inhibited (Marks et al., 1993) by nicotine treatment.
Upregulation can be induced in vitro by exposing oocytes or
cell lines expressing ␣42 nAChRs to chronic concentrations of
nicotine (Peng et al., 1994; Hsu et al., 1996; Gopalakrishnan et
al., 1997; Whiteaker et al., 1998). However, despite multiple
investigations, what is still unclear is whether upregulation results
in a functional increase or decrease and the relevance of these
mechanisms in nicotine addiction.
In vitro electrophysiological measurements have demonstrated
that prolonged ACh or nicotine applications (in a time scale of
minutes) produced a progressive decline of the current carried by
nAChRs (Katz and Thesleff, 1957; Peng et al., 1994; Lester and
Dani, 1995; Fenster et al., 1997; Pidoplichko et al., 1997; Corrin-
ger et al., 1998). Called “desensitization,” this decline corre-
sponds to a progressive closure of the receptors that are contin-
uously exposed to nicotinic agonists. On one hand, it has been
shown with the oocyte system that upregulation of ␣42 nAChRs
occurs after receptor desensitization (Peng et al., 1994; Fenster et
al., 1999a,b). On the other hand, Gopalakrishnan et al. (1996,
1997) have suggested that human ␣42 nAChRs expressed in
human embryonic kidney (HEK) 293 cells could be functional
after chronic exposure to nicotine or nicotinic ligands.
MATERIALS AND METHODS
K-177 is a stable cell line (HEK-293) expressing the human ␣4 and 2
nAChR subunits that was kindly provided by Abbott Laboratories (Chi-
cago, IL). Constructions of cDNAs, transfection procedures, selection,
Received June 30, 2000; revised Dec. 18, 2000; accepted Dec. 22, 2000.
This work was supported by the Swiss National Science Foundation and the
“Office Fe´de´ral de l’Education et des Sciences” (D. B.). We thank S. Bertrand, I.
Favre, L. Curtis, C. Blanchet, C. Yamate-Poitry, and J. Sullivan for their help and
discussions in the preparation of this manuscript. K-177 cells were kindly provided
by J. Sullivan (Abbott Laboratories, Chicago, IL).
Correspondence should be addressed to Daniel Bertrand, Department of Physi-
ology, Medical Faculty, 1 rue Michel Servet, 1211 Geneva 4, Switzerland. E-mail:
bertrand@cmu.unige.ch.
Dr. Buisson’s present address: De´partement de Screening, Trophos, Parc Scien-
tifique de Luminy, case 931, 13288 Marseille cedex 9, France.
Copyright © 2001 Society for Neuroscience 0270-6474/01/211819-11$15.00/0
The Journal of Neuroscience, March 15, 2001, 21(6):1819–1829](https://image.slidesharecdn.com/ab9cb85f-7e8f-40b7-9d4d-50bcaf33225d-150515065559-lva1-app6891/75/upregulHuman4b2nAChR-1-2048.jpg)


![these factors could provide further insights into the mechanisms
that modulate the upregulation of ␣42 nAChRs.
Because competitive antagonists induce upregulation of ␣42
nAChRs, a transmembrane signal has to trigger the incorporation
of presynthesized receptors into the membrane, if we suppose
that upregulation corresponds to an increase of the receptor
number within the cell membrane (Wonnacott, 1990). Different
mechanisms could be proposed for the transduction mechanism.
The receptors could interact with cytoskeletal elements (Liu and
Berg, 1999; Shoop et al., 2000) or could be coupled with metabo-
tropic receptors such as those described recently for GABAA
receptors (Liu et al., 2000).
Recent studies indicate that after a long-term exposure to
nicotine, ␣4 and 2 mRNA levels do not change in the mouse
brain (Marks et al., 1992) and that ␣42 proteins remain constant
in the membrane of cell lines (Whiteaker et al., 1998; Vallejo et
al., 1999). Our data, together with other studies (Zwart and
Vijverberg, 1998; Marutle et al., 1999; Shafaee et al., 1999; Vallejo
et al., 1999; Covernton and Connolly, 2000), indicate that surface
␣42 nAChRs are distributed in at least two populations. In the
light of previous observations (Romanelli et al., 1988; Bhat et al.,
1994; Vallejo et al., 1999), we propose that long-term exposure to
nicotine could induce a fraction of low-affinity nAChRs to
isomerize into high-affinity nAChRs by slow conformational tran-
sitions (time scale in hours). Such a hypothesis has the advantage
of providing a coherent framework to explain the full reversibility
of the upregulation process.
The eventuality that intracellular high-affinity receptors could
be incorporated into the cell membrane cannot be ruled out with
the present study. Reversibility of upregulation (Fig. 5) supposes,
however, that newly incorporated nAChRs must be removed
preferentially or shut down selectively within a few hours after
the removal of the upregulating compound.
The small but consistent increase in the ACh-evoked current
observed in cells treated with the protein inhibitor cycloheximide
alone (Table 1) indicates a possible influence of this drug on the
receptor number or equilibrium. In agreement with this observa-
tion, a small increase in the fraction of the high-affinity compo-
nent was also observed. Although presently unexplained, this
observation suggests a possible role of the protein synthesis in the
regulation of the receptor at the cell surface.
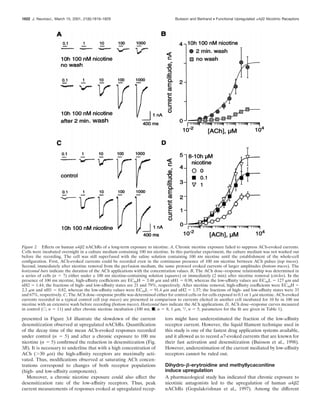
Detailed examination of the amount of current carried by the
high-affinity component between control and DHE-treated cells
revealed a 7.6-fold increase, whereas an increase of only threefold
of the open probability was observed in corresponding outside-
out patches. This would suggest that the number of highly acti-
vatable receptors must have at least doubled. That the amount of
current carried by the low-affinity component remains approxi-
mately constant makes it tempting to conclude that new receptors
must have been inserted into the membrane. Because of technical
limitations (channel rundown), this important point cannot be
resolved at present by electrophysiology alone. Therefore, further
measurements are necessary before a final conclusion can be
reached.
Our observations raise a fundamental question concerning the
properties of native ␣42 nAChRs. It is conceivable that in the
brain, a chronic low concentration of ACh could displace the
equilibrium between low- and high-affinity ␣42 nAChRs. This
mechanism could provide a molecular basis for the so-called
“volume transmission” (Agnati et al., 1995; Bertrand and Chan-
geux, 1995); that is, a neurotransmitter may diffuse in the extra-
cellular space and act on receptors present outside the synaptic
process. Moreover, we reveal that in vitro upregulation induces
ACh-evoked currents of higher amplitudes that desensitize more
slowly. Consistent with this observation, ␣42 nAChRs present in
presynaptic or postsynaptic membranes could promote enhanced
synaptic transmissions after their upregulation by low concentra-
tions of ACh or nicotine. Consequently, ␣42-linked pathologies,
such as autosomal dominant nocturnal frontal lobe epilepsy,
might be investigated within this new paradigm (Kuryatov et al.,
1997; Bertrand et al., 1998; Bertrand, 1999). Finally, pharmaco-
logical strategies targeted to ␣42 nAChRs (Lloyd and Williams,
2000) might take into account the unexpected properties of this
receptor subtype.
REFERENCES
Albuquerque EX, Pereira EF, Castro NG, Alkondon M, Reinhardt S,
Schroder H, Maelicke A (1995) Nicotinic receptor function in the
mammalian central nervous system. Ann NY Acad Sci 757:48–72.
Alkondon M, Albuquerque EX (1993) Diversity of nicotinic acetylcho-
line receptors in rat hippocampal neurons. I. Pharmacological and
functional evidence for distinct structural subtypes. J Pharmacol Exp
Ther 265:1455–1473.
Alkondon M, Pereira EF, Eisenberg HM, Albuquerque EX (2000) Nic-
otinic receptor activation in human cerebral cortical interneurons: a
mechanism for inhibition and disinhibition of neuronal networks.
J Neurosci 20:66–75.
Badio B, Daly JW (1994) Epibatidine, a potent analgetic and nicotinic
agonist. Mol Pharmacol 45:563–569.
Balfour DJ, Wright AE, Benwell ME, Birrell CE (2000) The putative
role of extra-synaptic mesolimbic dopamine in the neurobiology of
nicotine dependence. Behav Brain Res 113:73–83.
Ballivet M, Nef P, Couturier S, Rungger D, Bader CR, Bertrand D,
Cooper E (1988) Electrophysiology of a chick neuronal nicotinic ace-
tylcholine receptor expressed in Xenopus oocytes after cDNA injection.
Neuron 1:847–852.
Benwell ME, Balfour DJ, Birrell CE (1995) Desensitization of the
nicotine-induced mesolimbic dopamine responses during constant in-
fusion with nicotine. Br J Pharmacol 114:454–460.
Bertrand D, Ballivet M, Rungger D (1990) Activation and blocking of
neuronal nicotinic acetylcholine receptor reconstituted in Xenopus oo-
cytes. Proc Natl Acad Sci USA 87:1993–1997.
Bhat RV, Marks MJ, Collins AC (1994) Effects of chronic nicotine
infusion on kinetics of high-affinity nicotine binding. J Neurochem
62:574–581.
Buisson B, Bertrand D (1998) Open-channel blockers at the human
␣42 neuronal nicotinic acetylcholine receptor. Mol Pharmacol
53:555–563.
Buisson B, Gopalakrishnan M, Arneric SP, Sullivan JP, Bertrand D
(1996) Human ␣42 neuronal nicotinic acetylcholine receptor in HEK
293 cells: a patch-clamp study. J Neurosci 16:7880–7891.
Buisson B, Gopalakrishnan M, Bertrand D (1998) Stable expression of
human neuronal nicotinic receptors. In: Neuronal nicotinic receptors:
pharmacology and therapeutic opportunities (Arneric SP, Brioni JD,
eds), pp 99–124. New York: Wiley.
Buisson B, Vallejo YF, Green WN, Bertrand D (2000) The unusual
nature of epibatidine responses at the ␣42 nicotinic acetylcholine
receptor. Neuropharmacology 39:2561–2569.
Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela
CF, Harris RA (1999) Effects of ethanol on recombinant human neu-
ronal nicotinic acetylcholine receptors expressed in Xenopus oocytes.
J Pharmacol Exp Ther 289:774–780.
Changeux JP, Bertrand D, Corringer PJ, Dehaene S, Edelstein S, Lena C,
Le Novere N, Marubio L, Picciotto M, Zoli M (1998) Brain nicotinic
receptors: structure and regulation, role in learning and reinforcement.
Brain Res Brain Res Rev 26:198–216.
Charnet P, Labarca C, Cohen BN, Davidson N, Lester HA, Pilar G
(1992) Pharmacological and kinetic properties of alpha 4 beta 2 neu-
ronal nicotinic acetylcholine receptors expressed in Xenopus oocytes.
J Physiol (Lond) 450:375–394.
Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ,
Johnson EC (1997) Pharmacological characterization of recombinant
human neuronal nicotinic acetylcholine receptors h ␣ 2  2, h ␣ 2  4,
h ␣ 3  2, h ␣ 3  4, h ␣ 4  2, h ␣ 4  4 and h ␣ 7 expressed in Xenopus
oocytes. J Pharmacol Exp Ther 280:346–356.
Clarke PB, Schwartz RD, Paul SM, Pert CB, Pert A (1985) Nicotinic
binding in rat brain: autoradiographic comparison of [3H]acetylcho-
line, [3H]nicotine, and [125I]-␣-bungarotoxin. J Neurosci 5:1307–1315.
Conroy WG, Berg DK (1998) Nicotinic receptor subtypes in the devel-
oping chick brain: appearance of a species containing the ␣4, 2, and ␣5
gene products. Mol Pharmacol 53:392–401.
Buisson and Bertrand • Functional Upregulated ␣42 Nicotinic Receptors J. Neurosci., March 15, 2001, 21(6):1819–1829 1827](https://image.slidesharecdn.com/ab9cb85f-7e8f-40b7-9d4d-50bcaf33225d-150515065559-lva1-app6891/85/upregulHuman4b2nAChR-9-320.jpg)
![Cooper E, Couturier S, Ballivet M (1991) Pentameric structure and
subunit stoichiometry of a neuronal nicotinic acetylcholine receptor.
Nature 350:235–238.
Corringer PJ, Bertrand S, Bohler S, Edelstein SJ, Changeux JP, Bertrand
D (1998) Critical elements determining diversity in agonist binding
and desensitization of neuronal nicotinic acetylcholine receptors.
J Neurosci 18:648–657.
Covernton PJ, Connolly JG (2000) Multiple components in the agonist
concentration-response relationships of neuronal nicotinic acetylcho-
line receptors. J Neurosci Methods 96:63–70.
Creese I, Sibley DR (1981) Receptor adaptations to centrally acting
drugs. Annu Rev Pharmacol Toxicol 21:357–391.
Dani JA, Heinemann S (1996) Molecular and cellular aspects of nicotine
abuse. Neuron 16:905–908.
Derkach V, Barria A, Soderling TR (1999) Ca2ϩ/calmodulin-kinase II
enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-
isoxazolepropionate type glutamate receptors. Proc Natl Acad Sci USA
96:3269–3274.
Deutch AY, Holliday J, Roth RH, Chun LL, Hawrot E (1987) Immu-
nohistochemical localization of a neuronal nicotinic acetylcholine re-
ceptor in mammalian brain. Proc Natl Acad Sci USA 84:8697–8701.
Fenster CP, Rains MF, Noerager B, Quick MW, Lester RA (1997)
Influence of subunit composition on desensitization of neuronal ace-
tylcholine receptors at low concentrations of nicotine. J Neurosci
17:5747–5759.
Fenster CP, Beckman ML, Parker JC, Sheffield EB, Whitworth TL, Quick
MW, Lester RA (1999a) Regulation of ␣42 nicotinic receptor de-
sensitization by calcium and protein kinase C. Mol Pharmacol
55:432–443.
Fenster CP, Whitworth TL, Sheffield EB, Quick MW, Lester RA (1999b)
Upregulation of surface ␣42 nicotinic receptors is initiated by recep-
tor desensitization after chronic exposure to nicotine. J Neurosci
19:4804–4814.
Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ (1992) A
subtype of nicotinic cholinergic receptor in rat brain is composed of
alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine
treatment. Mol Pharmacol 41:31–37.
Flores CM, Davila-Garcia MI, Ulrich YM, Kellar KJ (1997) Differential
regulation of neuronal nicotinic receptor binding sites following
chronic nicotine administration. J Neurochem 69:2216–2219.
Gamberino WC, Gold MS (1999) Neurobiology of tobacco smoking and
other addictive disorders. Psychiatr Clin North Am 22:301–312.
Gopalakrishnan M, Monteggia LM, Anderson DJ, Molinari EJ, Piattoni-
Kaplan M, Donnelly-Roberts D, Arneric SP, Sullivan JP (1996) Stable
expression, pharmacologic properties and regulation of the human
neuronal nicotinic acetylcholine alpha 4 beta 2 receptor. J Pharmacol
Exp Ther 276:289–297.
Gopalakrishnan M, Molinari EJ, Sullivan JP (1997) Regulation of hu-
man ␣42 neuronal nicotinic acetylcholine receptors by cholinergic
channel ligands and second messenger pathways. Mol Pharmacol 52:
524–534.
Grottick AJ, Wyler R, Higgins GA (2000) The ␣42 agonist SIB 1765F,
but not the ␣7 agonist AR-R 17779, cross-sensitises to the psychostimu-
lant effects of nicotine. Psychopharmacology (Berl) 150:233–236.
Henningfield JE, Stapleton JM, Benowitz NL, Grayson RF, London ED
(1993) Higher levels of nicotine in arterial than in venous blood after
cigarette smoking. Drug Alcohol Depend 33:23–29.
Houghtling RA, Davila-Garcia MI, Kellar KJ (1995) Characterization
of (ϩ/Ϫ)(Ϫ)[3H]epibatidine binding to nicotinic cholinergic receptors
in rat and human brain. Mol Pharmacol 48:280–287.
Hsu YN, Amin J, Weiss DS, Wecker L (1996) Sustained nicotine expo-
sure differentially affects alpha 3 beta 2 and alpha 4 beta 2 neuronal
nicotinic receptors expressed in Xenopus oocytes. J Neurochem
66:667–675.
Katz B, Thesleff S (1957) A study of the desensitization produced by
acetylcholine at the motor end-plate. J Physiol (Lond) 138:63–80.
Koylu E, Demirgoren S, London ED, Pogun S (1997) Sex difference in
up-regulation of nicotinic acetylcholine receptors in rat brain. Life Sci
61:185–190.
Kuryatov A, Gerzanich V, Nelson M, Olale F, Lindstrom J (1997) Mu-
tation causing autosomal dominant nocturnal frontal lobe epilepsy
alters Ca2ϩ
permeability, conductance, and gating of human ␣42
nicotinic acetylcholine receptors. J Neurosci 17:9035–9047.
Lapchak PA, Araujo DM, Quirion R, Collier B (1989) Effect of chronic
nicotine treatment on nicotinic autoreceptor function and
N-[3H]methylcarbamylcholine binding sites in the rat brain. J Neuro-
chem 52:483–491.
Leshner AI, Koob GF (1999) Drugs of abuse and the brain. Proc Assoc
Am Physicians 111:99–108.
Lester RA, Dani JA (1995) Acetylcholine receptor desensitization in-
duced by nicotine in rat medial habenula neurons. J Neurophysiol
74:195–206.
Liu Q-S, Berg D (1999) Actin filaments and the opposing actions of CaM
kinase II and calcineurin in regulating ␣7-containing nicotinic recep-
tors on chick ciliary ganglion neurons. J Neurosci 19:10280–10288.
Marks MJ, Stitzel JA, Collins AC (1985) Time course study of the effects
of chronic nicotine infusion on drug response and brain receptors.
J Pharmacol Exp Ther 235:619–628.
Marks MJ, Pauly JR, Gross SD, Deneris ES, Hermans-Borgmeyer I,
Heinemann SF, Collins AC (1992) Nicotine binding and nicotinic
receptor subunit RNA after chronic nicotine treatment. J Neurosci
12:2765–2784.
Marks MJ, Grady SR, Collins AC (1993) Downregulation of nicotinic
receptor function after chronic nicotine infusion. J Pharmacol Exp
Ther 266:1268–1276.
Marks MJ, Whiteaker P, Calcaterra J, Stitzel JA, Bullock AE, Grady SR,
Picciotto MR, Changeux JP, Collins AC (1999) Two pharmacologi-
cally distinct components of nicotinic receptor-mediated rubidium ef-
flux in mouse brain require the 2 subunit. J Pharmacol Exp Ther
289:1090–1103.
Marubio LM, del Mar Arroyo-Jimenez M, Cordero-Erausquin M, Lena
C, Le Novere N, de Kerchove d’Exaerde A, Huchet M, Damaj MI,
Changeux JP (1999) Reduced antinociception in mice lacking neuro-
nal nicotinic receptor subunits. Nature 398:805–810.
Marutle A, Warpman U, Bogdanovic N, Lannfelt L, Nordberg A (1999)
Neuronal nicotinic receptor deficits in Alzheimer patients with the
Swedish amyloid precursor protein 670/671 mutation. J Neurochem
72:1161–1169.
Nelson ME, Kuryatov A, Lindstrom JM (1999) Host cell effects on
functional properties of human ␣42 nicotinic ACHRS. Soc Neurosci
Abstr 25:1722.
Papke RL, Boulter J, Patrick J, Heinemann S (1989) Single-channel
currents of rat neuronal nicotinic acetylcholine receptors expressed in
Xenopus oocytes. Neuron 3:589–596.
Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J (1994)
Nicotine-induced increase in neuronal nicotinic receptors results
from a decrease in the rate of receptor turnover. Mol Pharmacol
46:523–530.
Pereira EF, Alkondon M, Reinhardt S, Maelicke A, Peng X, Lindstrom
J, Whiting P, Albuquerque EX (1994) Physostigmine and galan-
thamine: probes for a novel binding site on the alpha 4 beta 2 subtype
of neuronal nicotinic acetylcholine receptors stably expressed in fibro-
blast cells. J Pharmacol Exp Ther 270:768–778.
Perry DC, Davila-Garcia MI, Stockmeier CA, Kellar KJ (1999) In-
creased nicotinic receptors in brains from smokers: membrane binding
and autoradiography studies. J Pharmacol Exp Ther 289:1545–1552.
Picciotto MR, Zoli M, Lena C, Bessis A, Lallemand Y, LeNovere N,
Vincent P, Pich EM, Brulet P, Changeux JP (1995) Abnormal avoid-
ance learning in mice lacking functional high-affinity nicotine receptor
in the brain. Nature 374:65–67.
Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM,
Fuxe K, Changeux JP (1998) Acetylcholine receptors containing the
beta2 subunit are involved in the reinforcing properties of nicotine.
Nature 391:173–177.
Pidoplichko VI, DeBiasi M, Williams JT, Dani JA (1997) Nicotine acti-
vates and desensitizes midbrain dopamine neurons. Nature
390:401–404.
Ragozzino D, Fucile S, Giovannelli A, Grassi F, Mileo AM, Ballivet M,
Alema S, Eusebi F (1997) Functional properties of neuronal nicotinic
acetylcholine receptor channels expressed in transfected human cells.
Eur J Neurosci 9:480–488.
Ramirez-Latorre J, Yu CR, Qu X, Perin F, Karlin A, Role L (1996)
Functional contributions of alpha5 subunit to neuronal acetylcholine
receptor channels. Nature 380:347–351.
Romanelli L, Ohman B, Adem A, Nordberg A (1988) Subchronic treat-
ment of rats with nicotine: interconversion of nicotinic receptor sub-
types in brain. Eur J Pharmacol 148:289–291.
Rowell PP, Wonnacott S (1990) Evidence for functional activity of up-
regulated nicotine binding sites in rat striatal synaptosomes. J Neuro-
chem 55:2105–2110.
Sabey K, Paradiso K, Zhang J, Steinbach JH (1999) Ligand binding and
activation of rat nicotinic ␣42 receptors stably expressed in HEK293
cells. Mol Pharmacol 55:58–66.
Schoepfer R, Whiting P, Esch F, Blacher R, Shimasaki S, Lindstrom J
(1988) cDNA clones coding for the structural subunit of a chicken
brain nicotinic acetylcholine receptor. Neuron 1:241–248.
Shafaee N, Houng M, Truong A, Viseshakul N, Figl A, Sandhu S,
Forsayeth JR, Dwoskin LP, Crooks PA, Cohen BN (1999) Pharmaco-
logical similarities between native brain and heterologously expressed
alpha4beta2 nicotinic receptors. Br J Pharmacol 128:1291–1299.
Sharples CG, Kaiser S, Soliakov L, Marks MJ, Collins AC, Washburn M,
Wright E, Spencer JA, Gallagher T, Whiteaker P, Wonnacott S (2000)
UB-165: a novel nicotinic agonist with subtype selectivity implicates the
␣42* subtype in the modulation of dopamine release from rat striatal
synaptosomes. J Neurosci 20:2783–2791.
Swanson LW, Simmons DM, Whiting PJ, Lindstrom J (1987) Immuno-
histochemical localization of neuronal nicotinic receptors in the rodent
central nervous system. J Neurosci 7:3334–3342.
1828 J. Neurosci., March 15, 2001, 21(6):1819–1829 Buisson and Bertrand • Functional Upregulated ␣42 Nicotinic Receptors](https://image.slidesharecdn.com/ab9cb85f-7e8f-40b7-9d4d-50bcaf33225d-150515065559-lva1-app6891/85/upregulHuman4b2nAChR-10-320.jpg)
