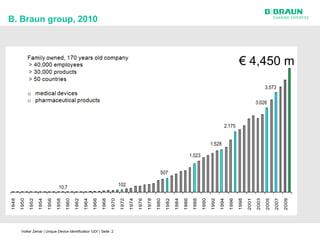
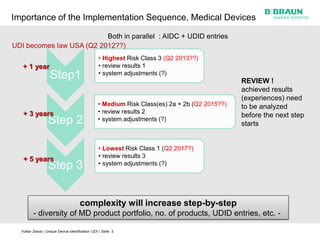
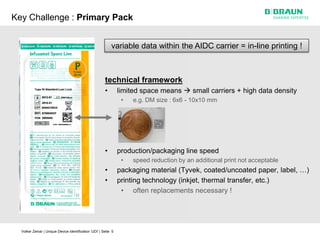
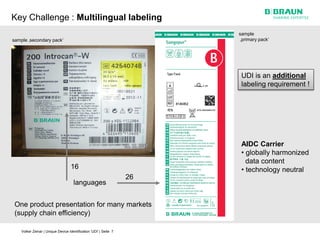
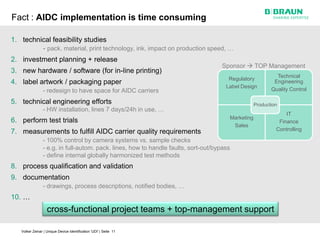
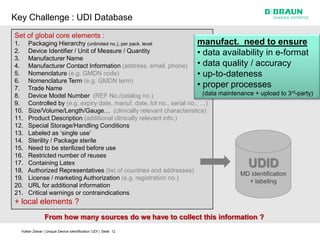
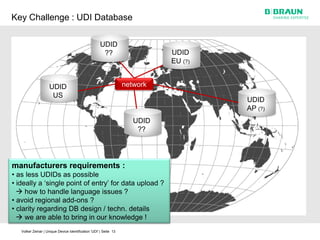
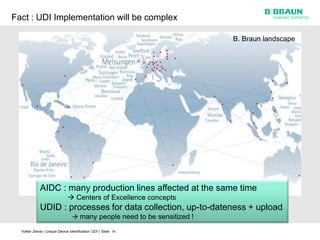
This document discusses the challenges medical device manufacturers will face in implementing Unique Device Identification (UDI). It argues that a step-wise implementation timeline is needed as UDI regulations will require adding variable data to automatic identification and data capture (AIDC) markings on individual medical device packages and products. This will be technically challenging due to limited space for small AIDC carriers, requirements to print variable data inline during high-speed packaging, and ensuring the quality and scannability of AIDC markings on different packaging materials. The document advocates for reviewing results between implementation steps to adjust systems as needed before expanding UDI to additional device classes.