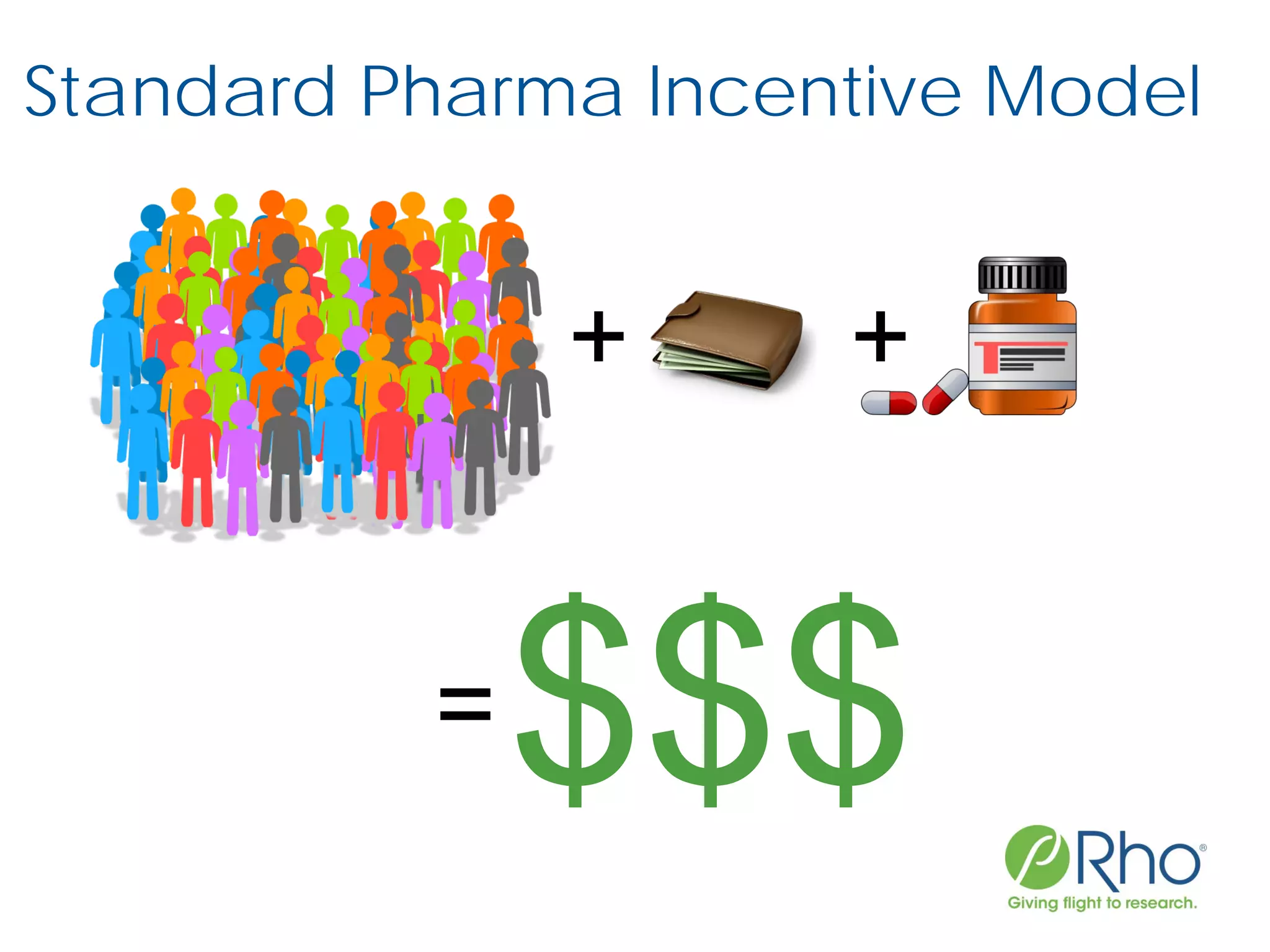
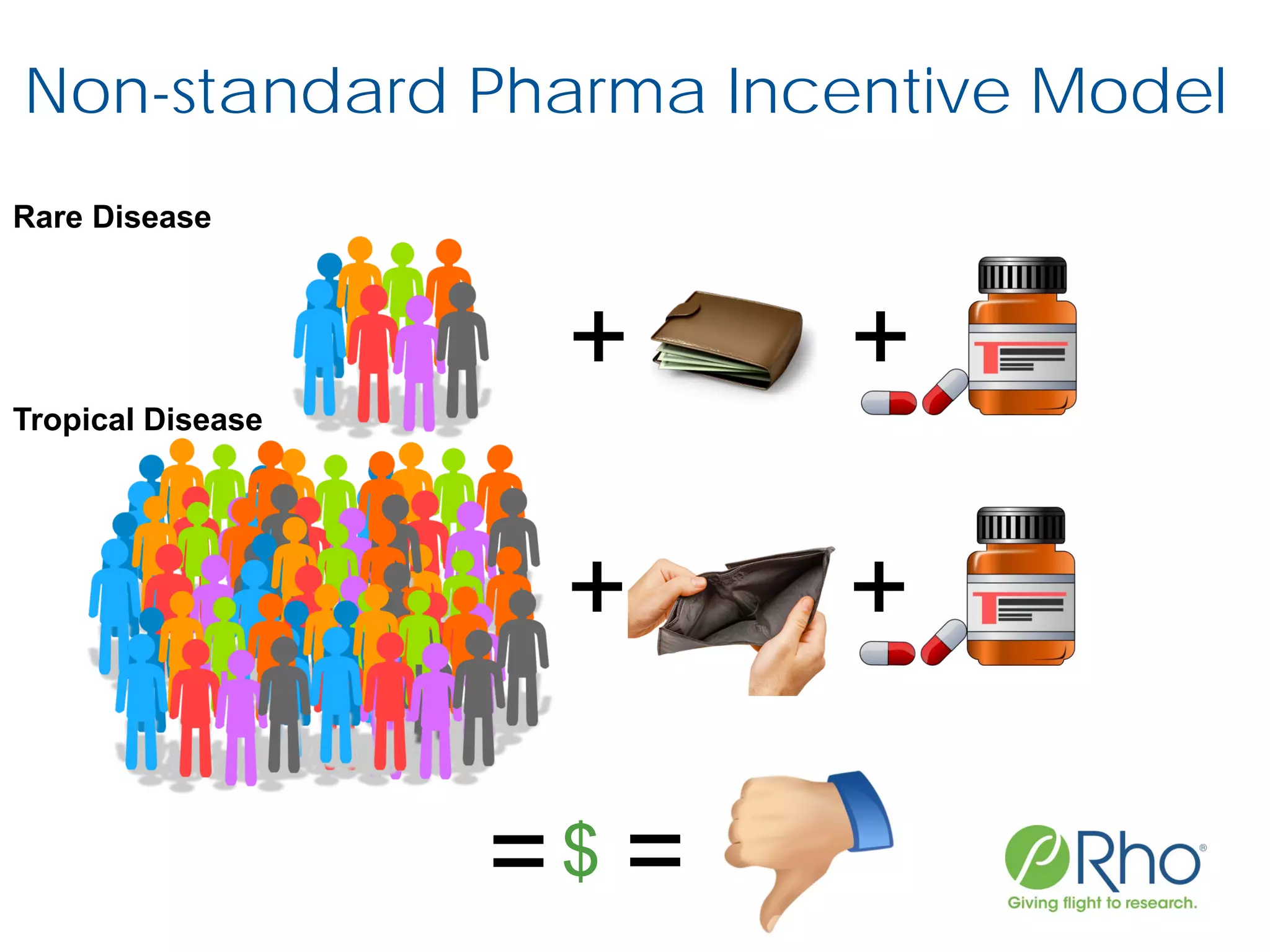
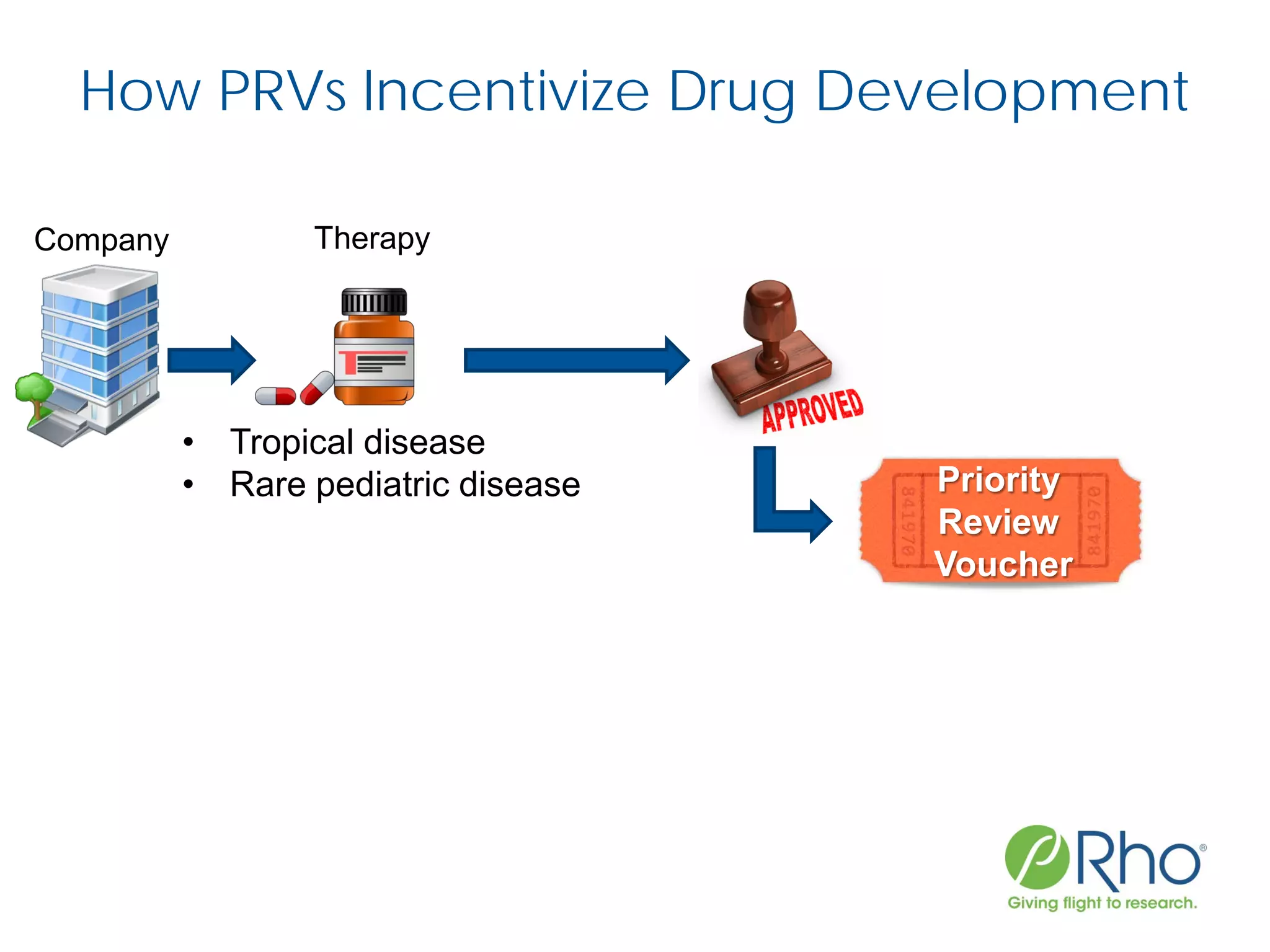
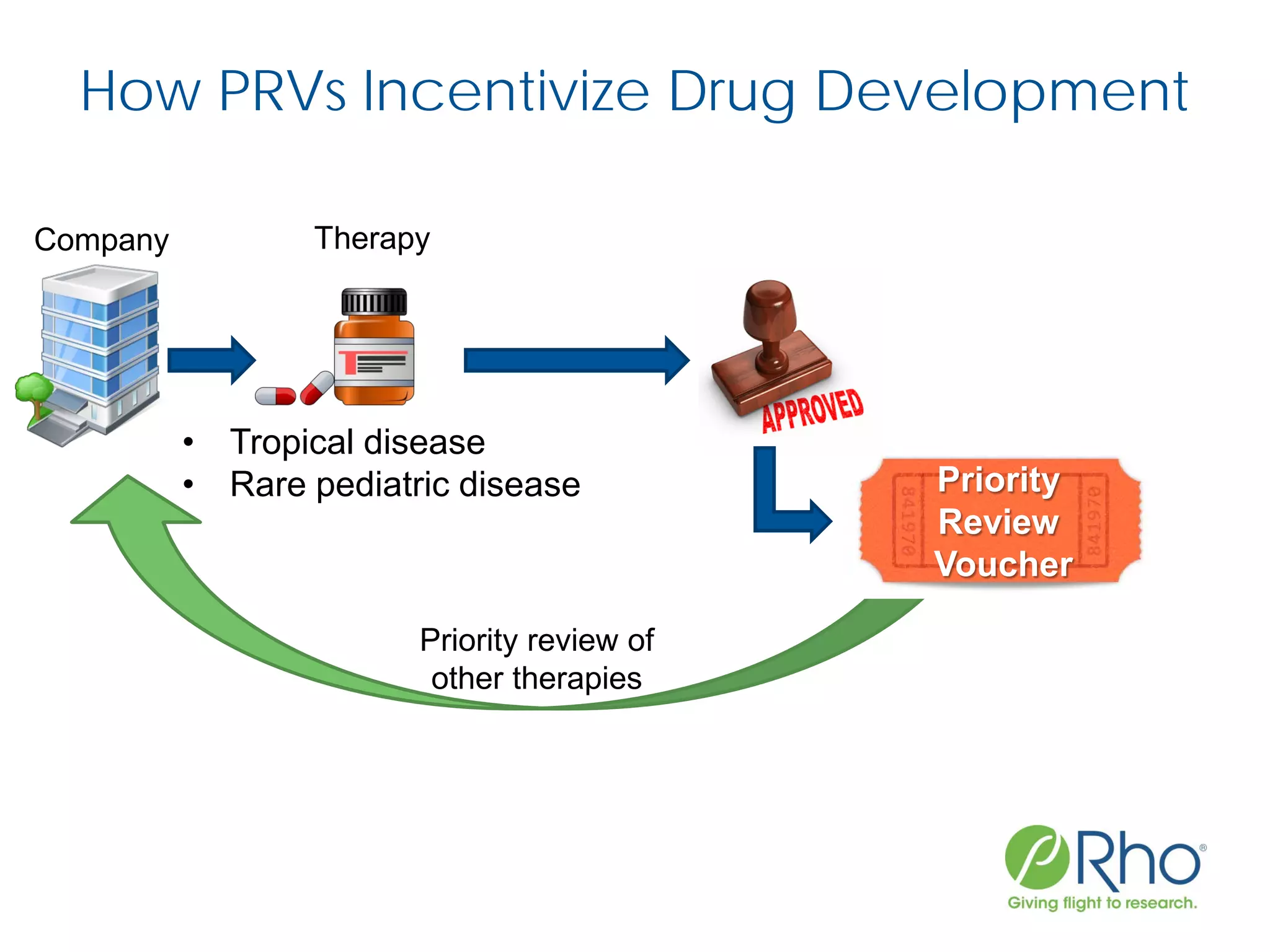
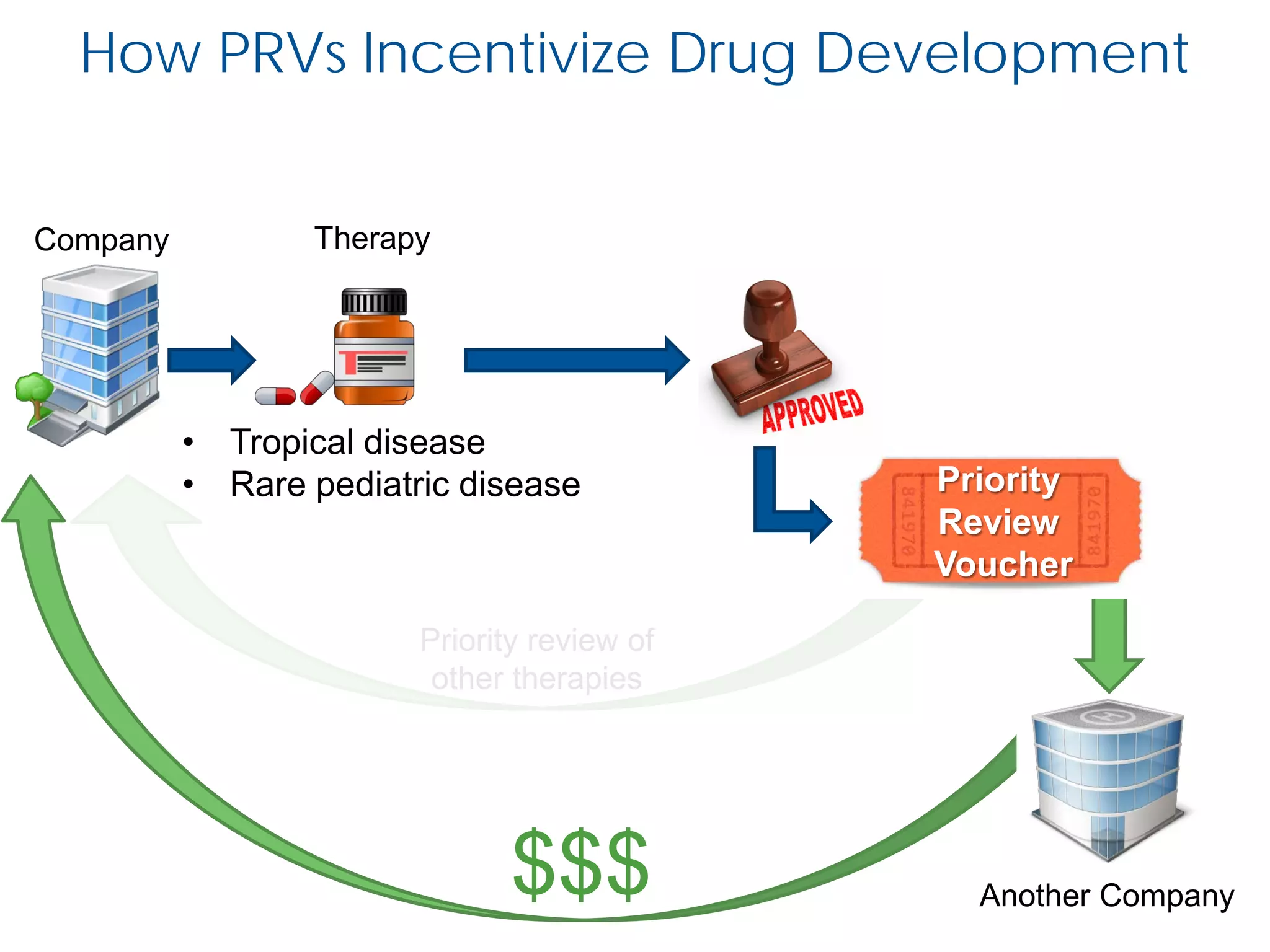
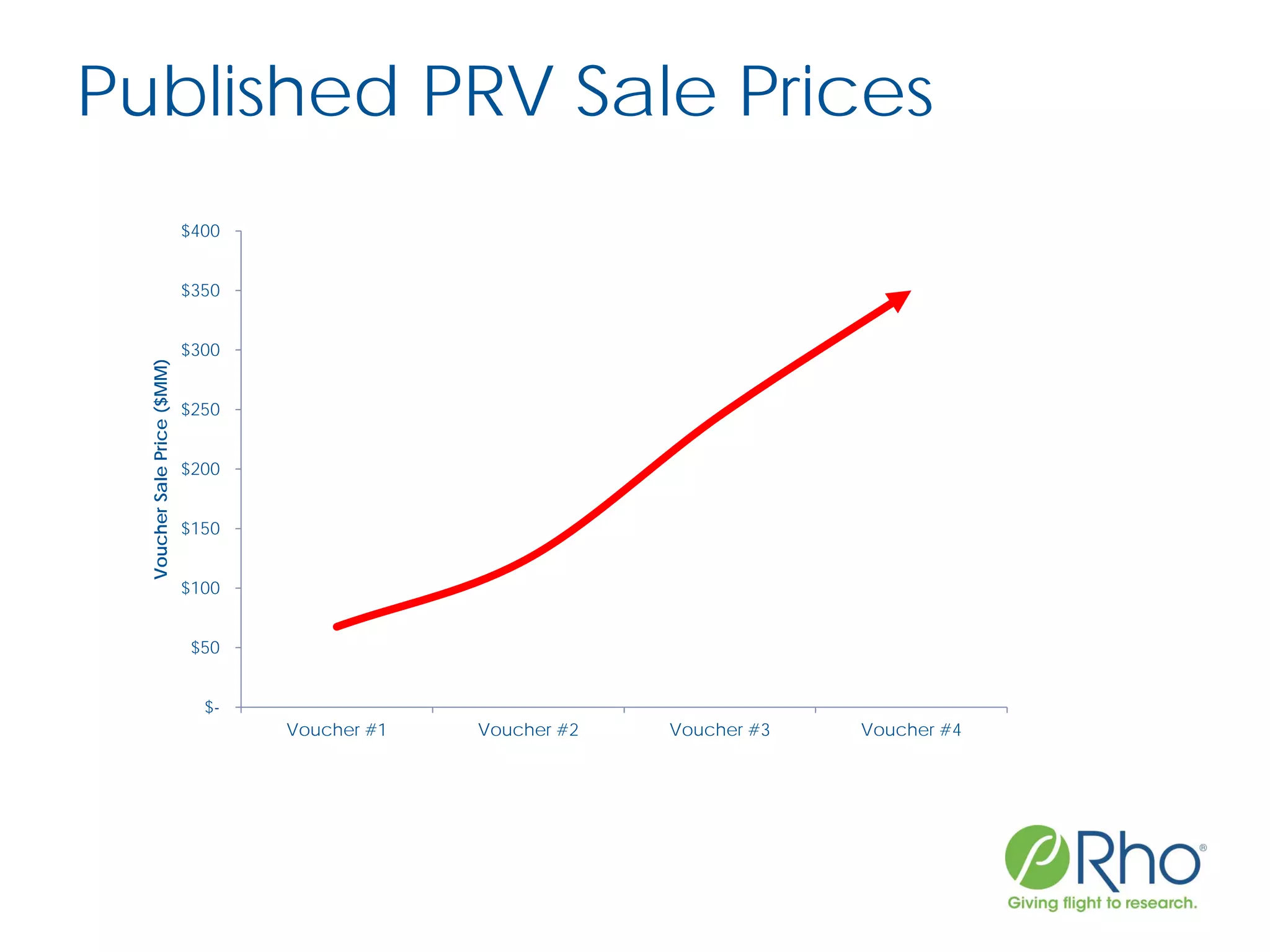
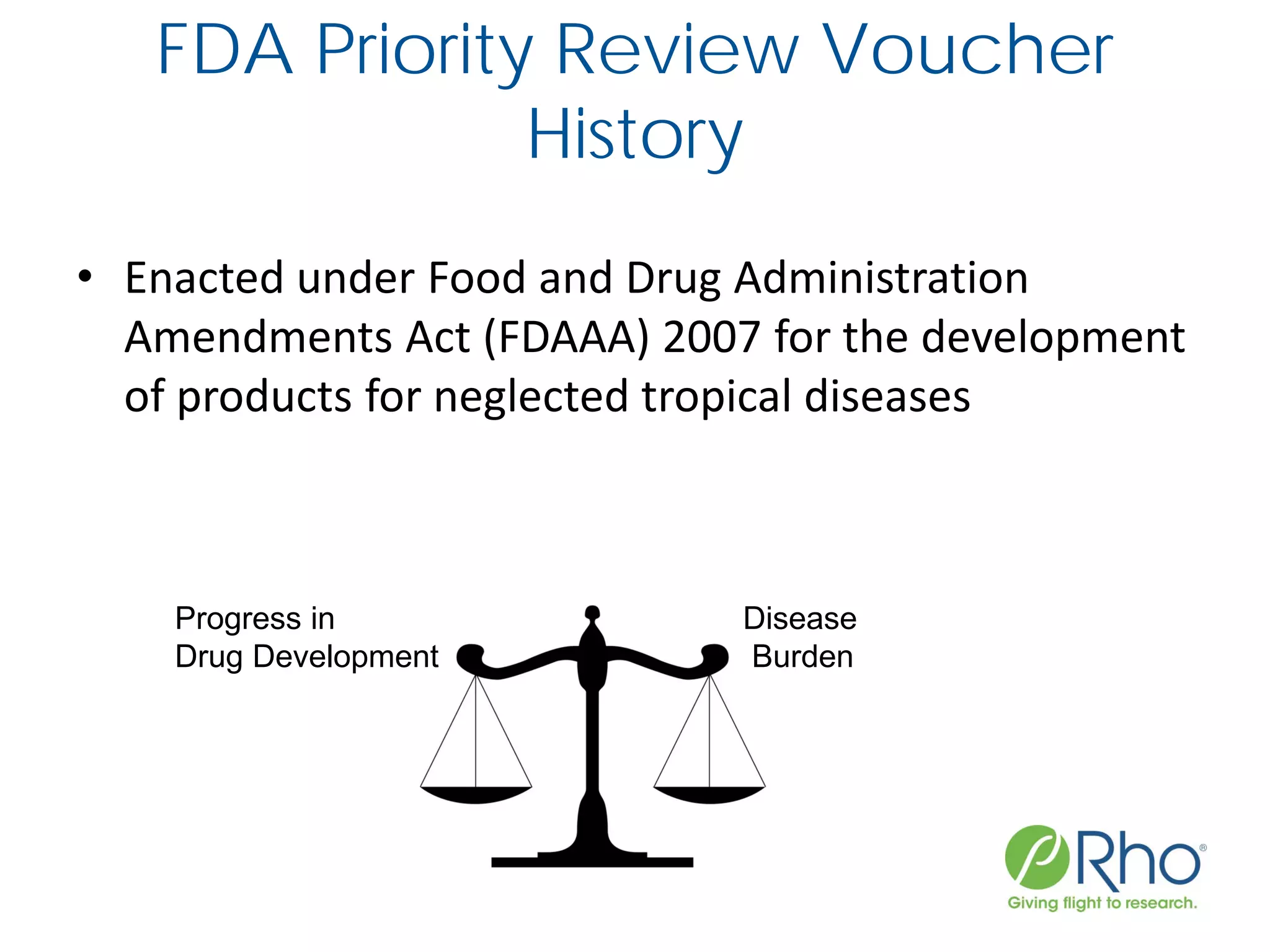
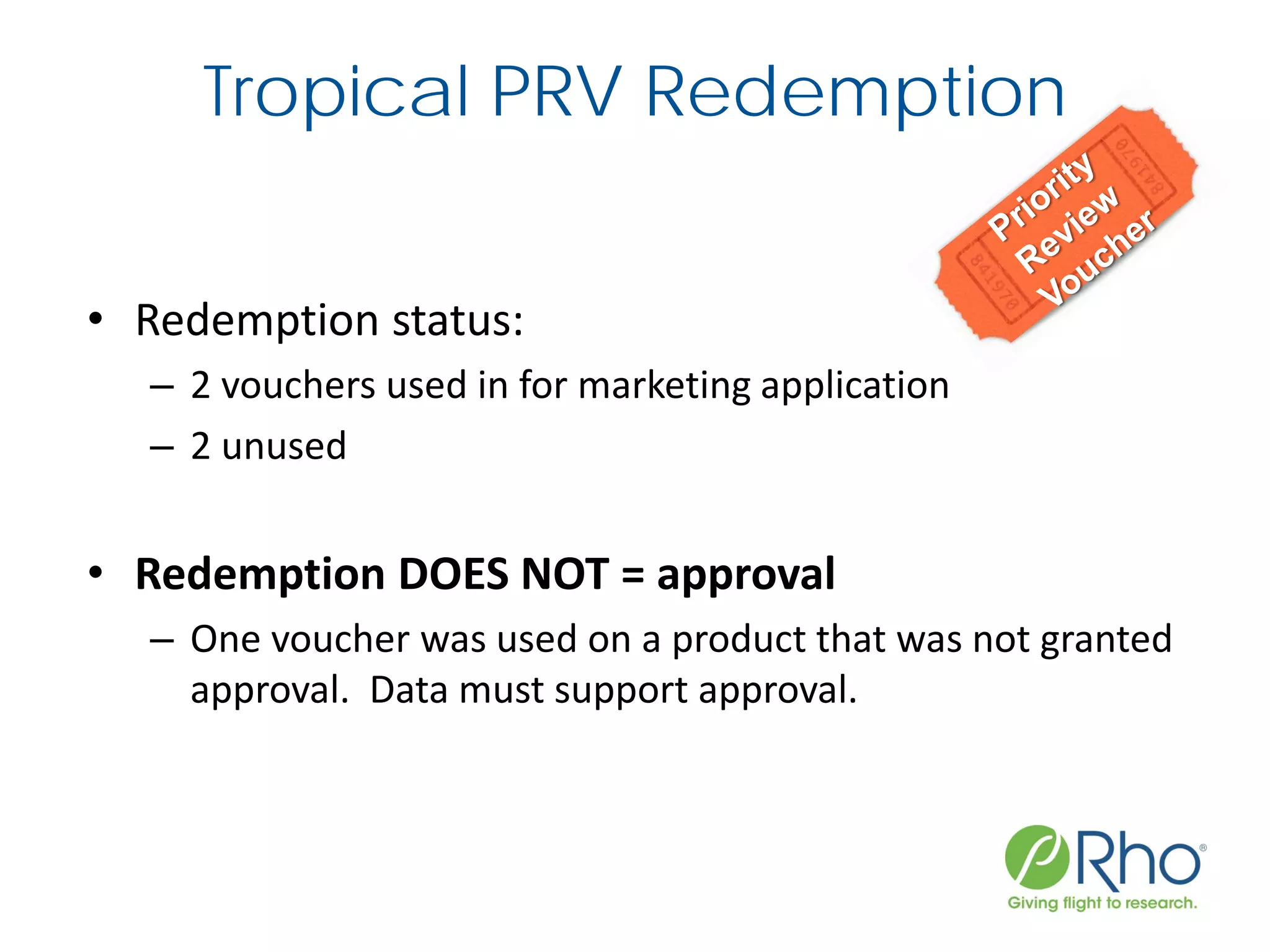
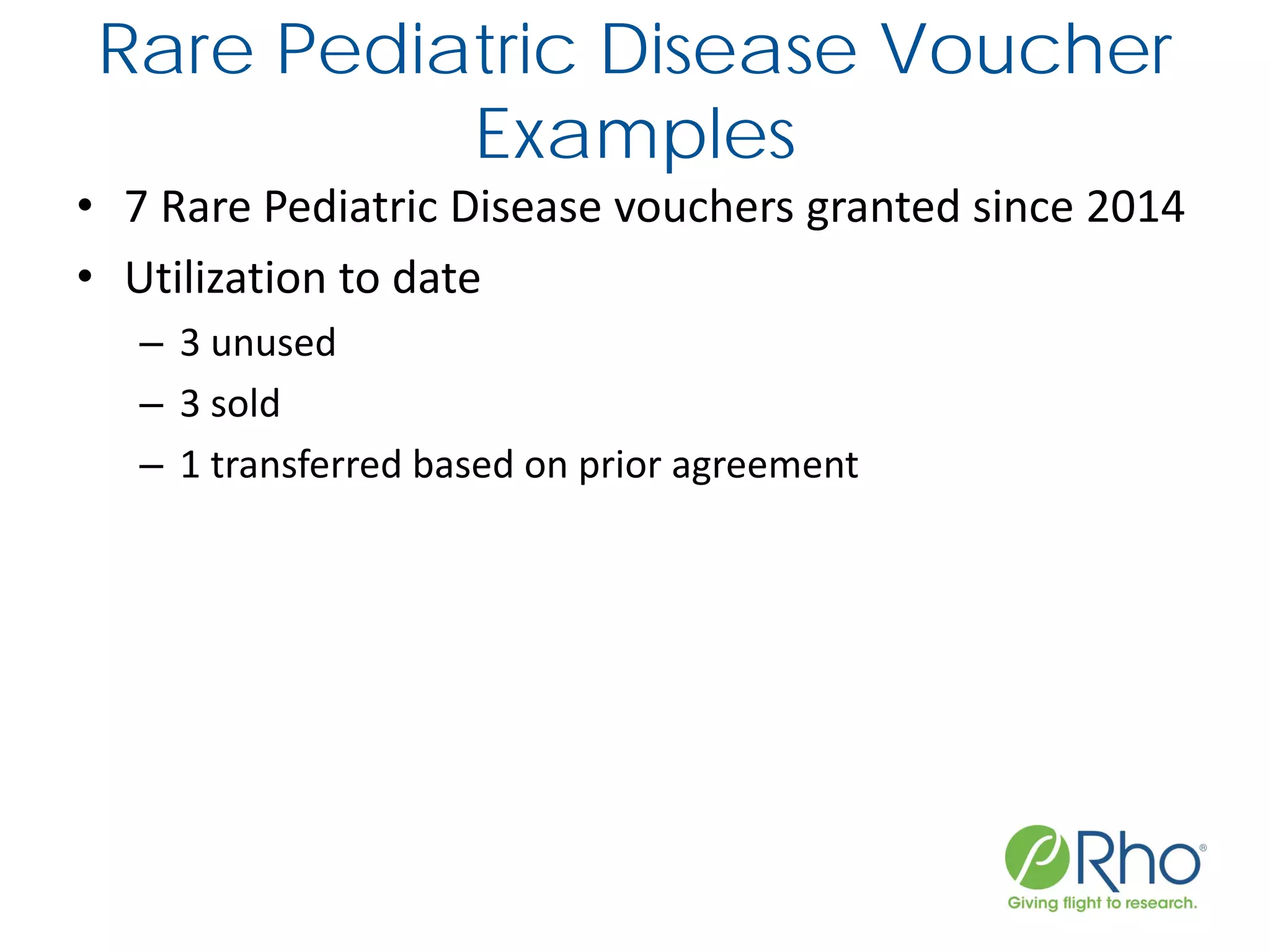
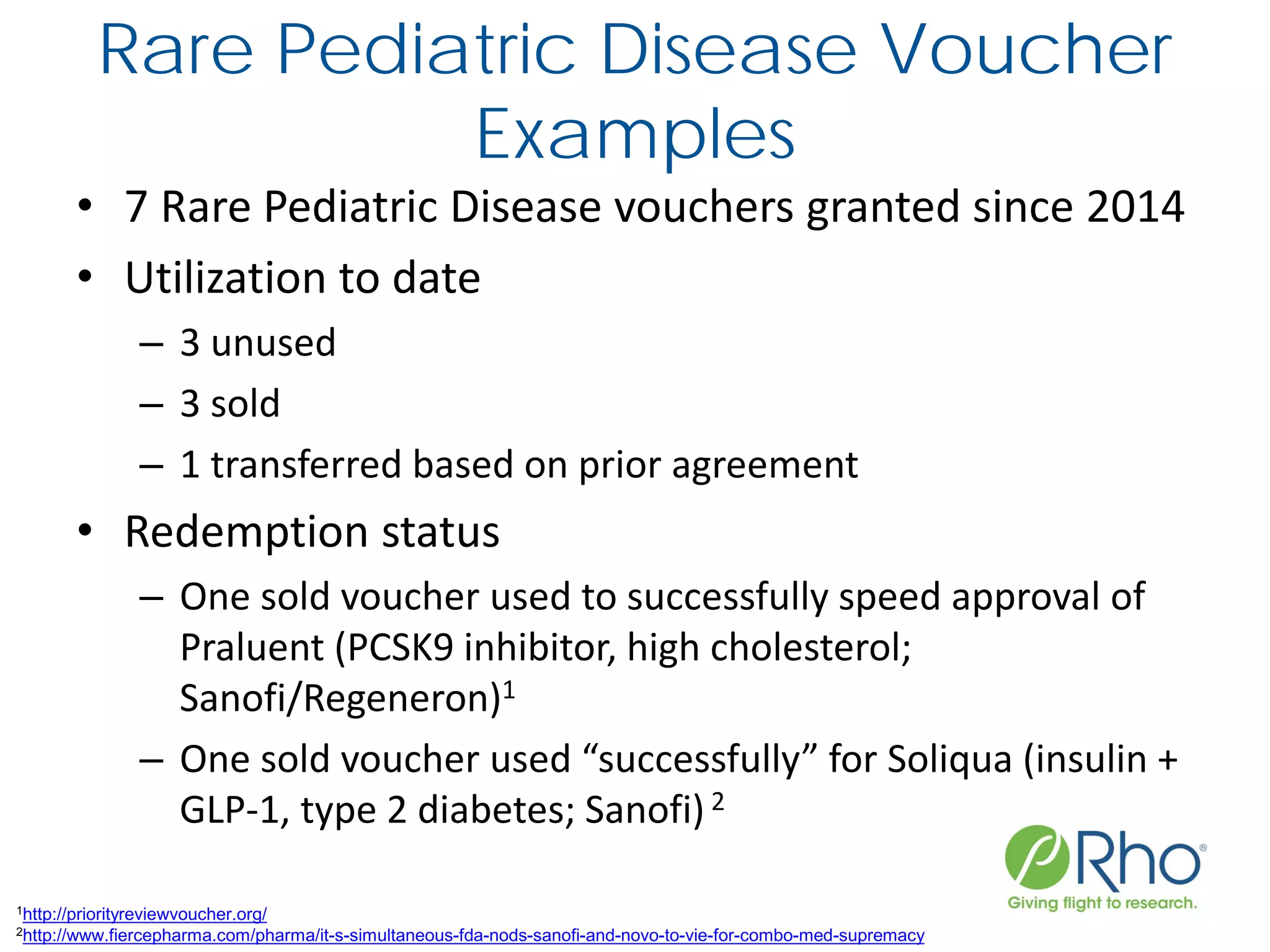
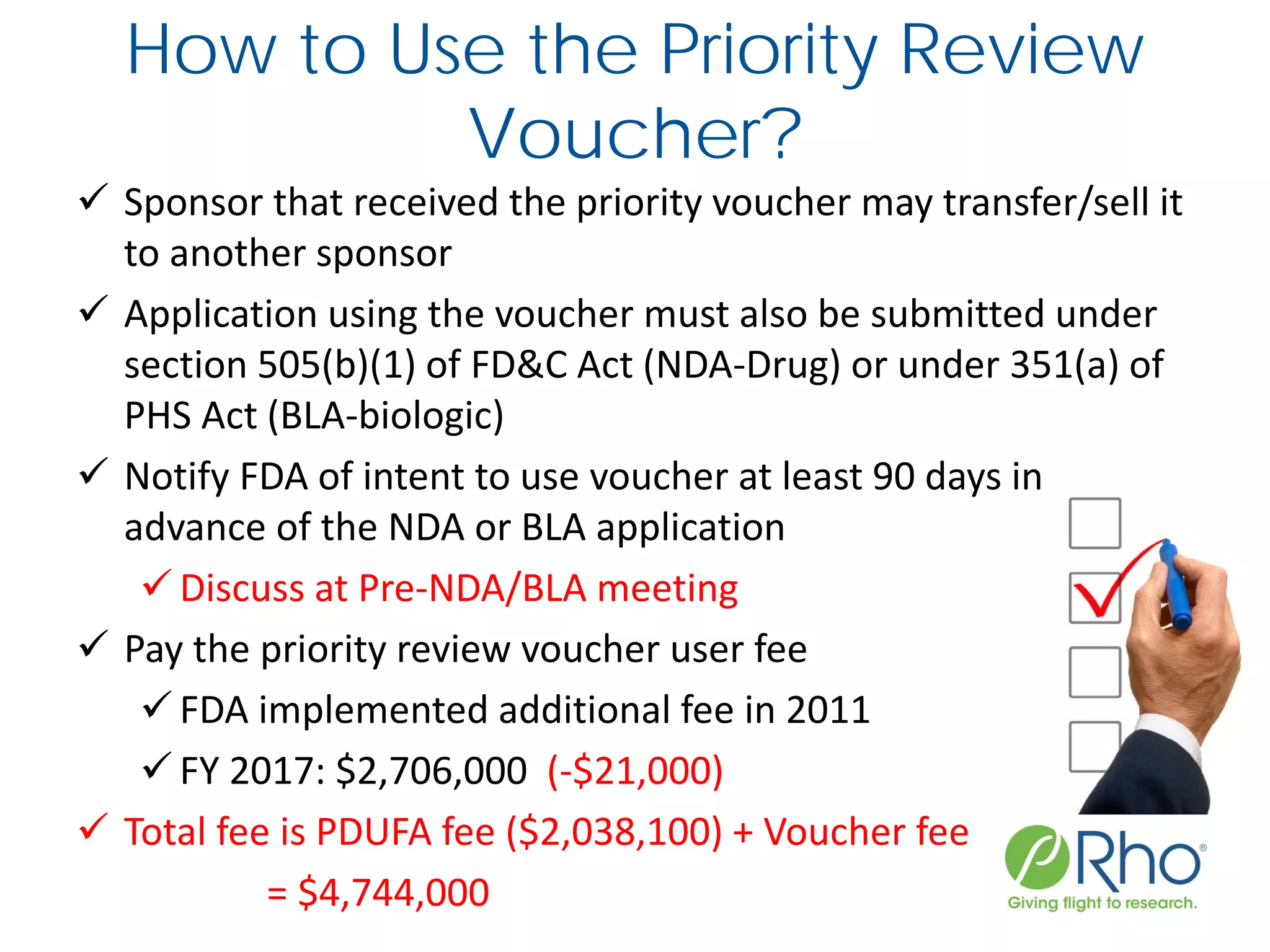
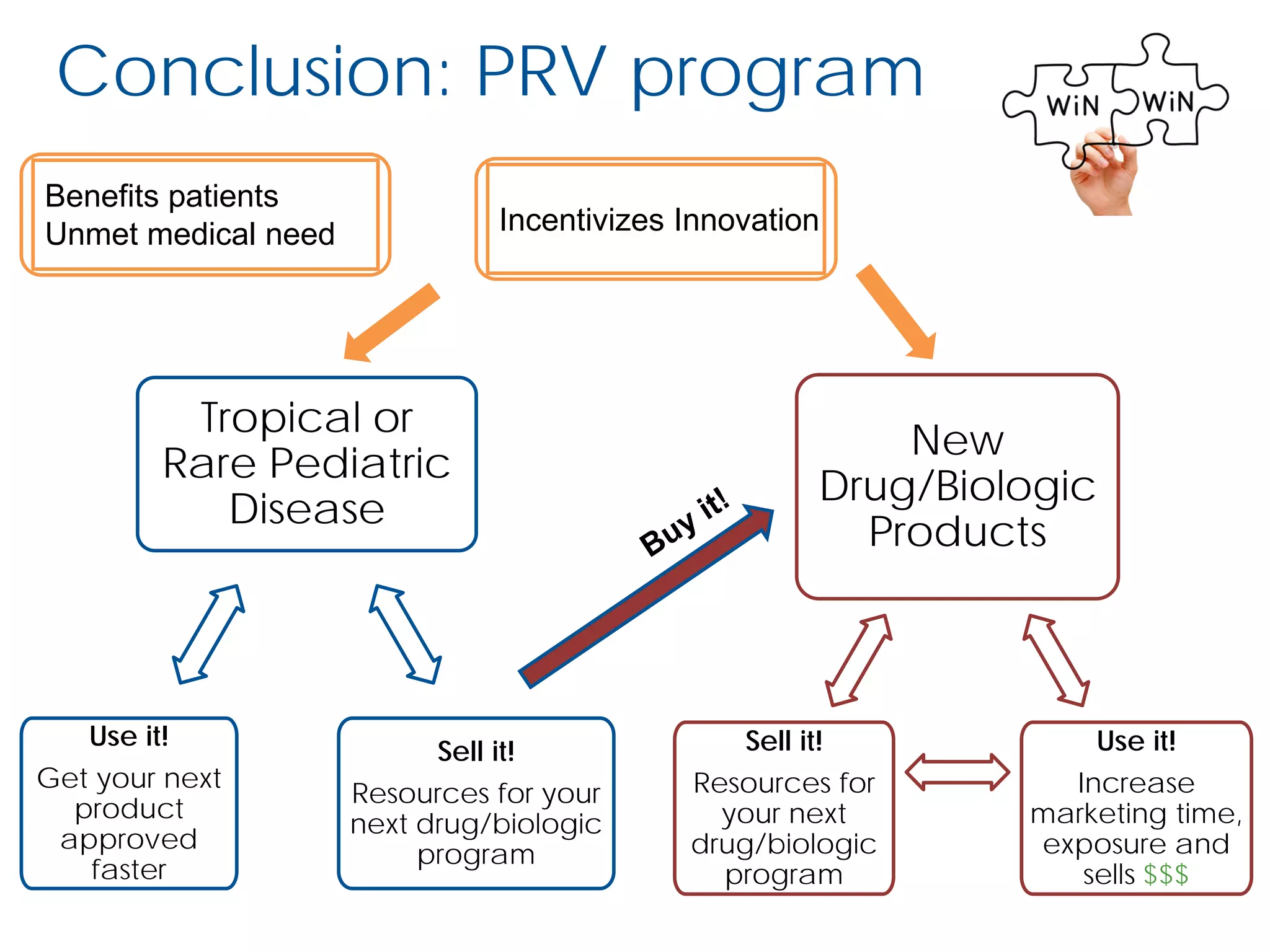
The document outlines the FDA’s Priority Review Voucher (PRV) system, which incentivizes the development of treatments for neglected tropical diseases and rare pediatric diseases by reducing the FDA's review time for drug applications. It details the eligibility criteria and application process for obtaining PRVs, alongside examples of successful vouchers and legislative changes affecting the program. The document also discusses challenges and limitations of the PRV program, expressing concerns about its effectiveness and proposing extensions for pediatric programs.