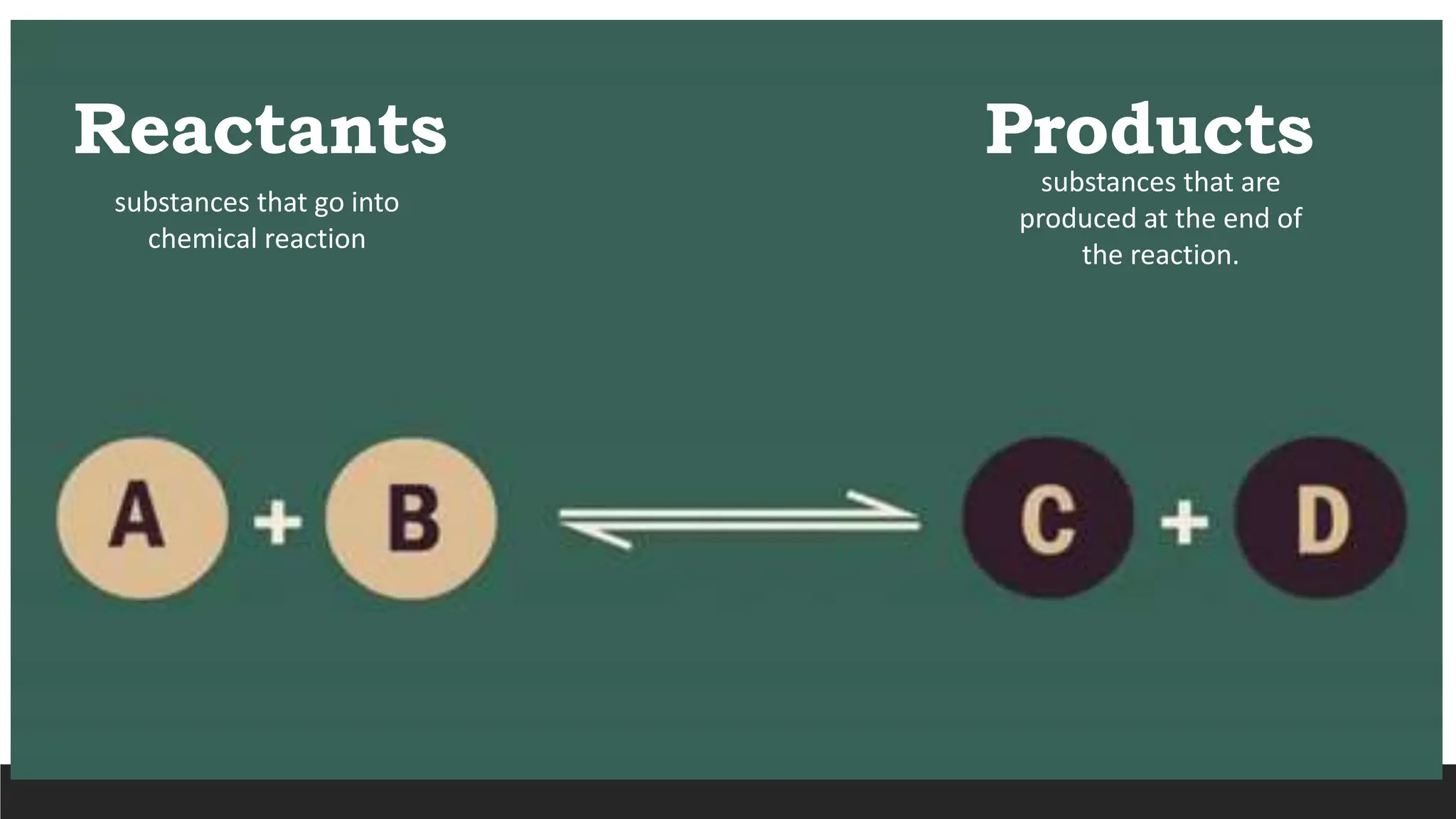
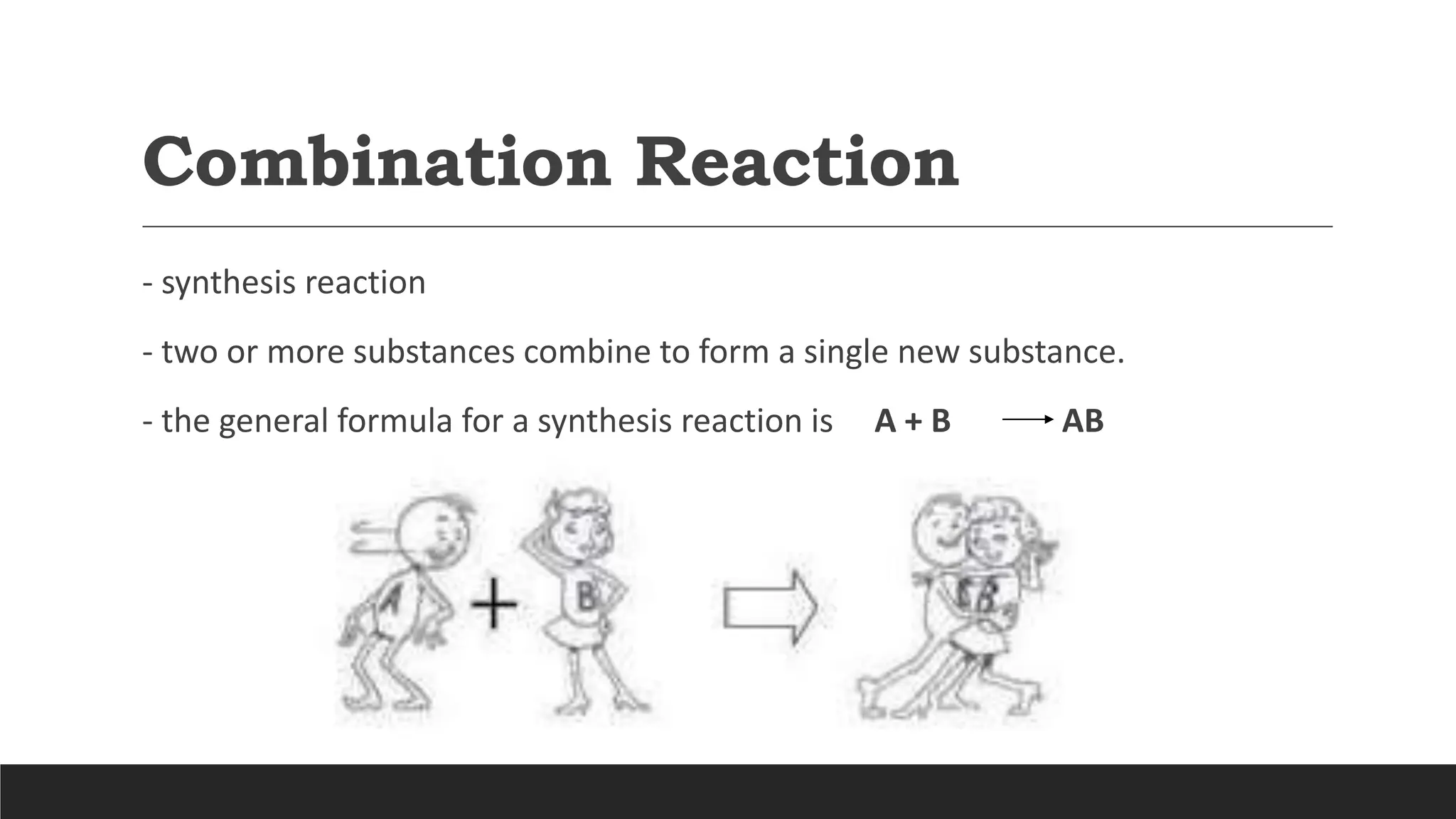
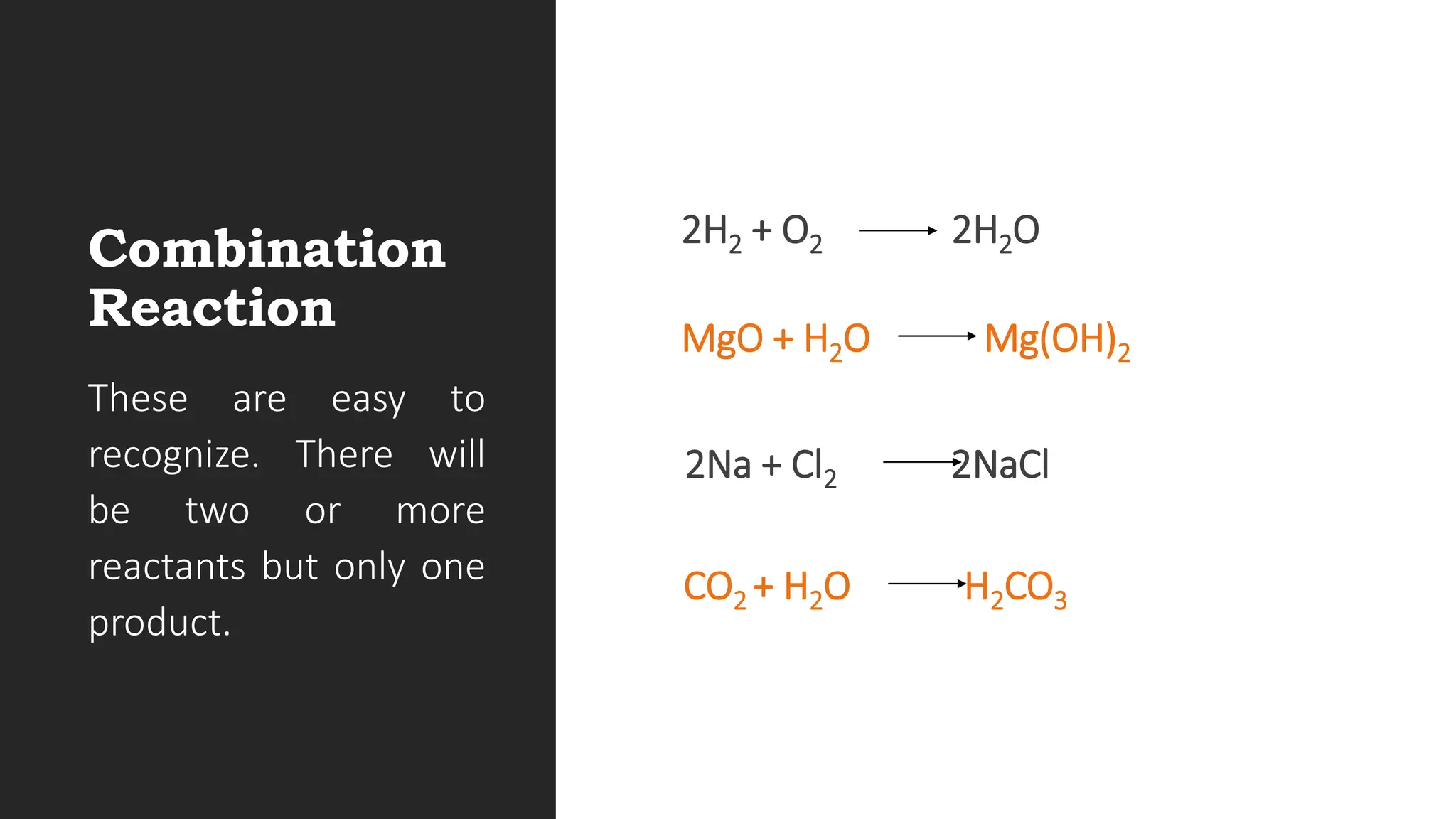
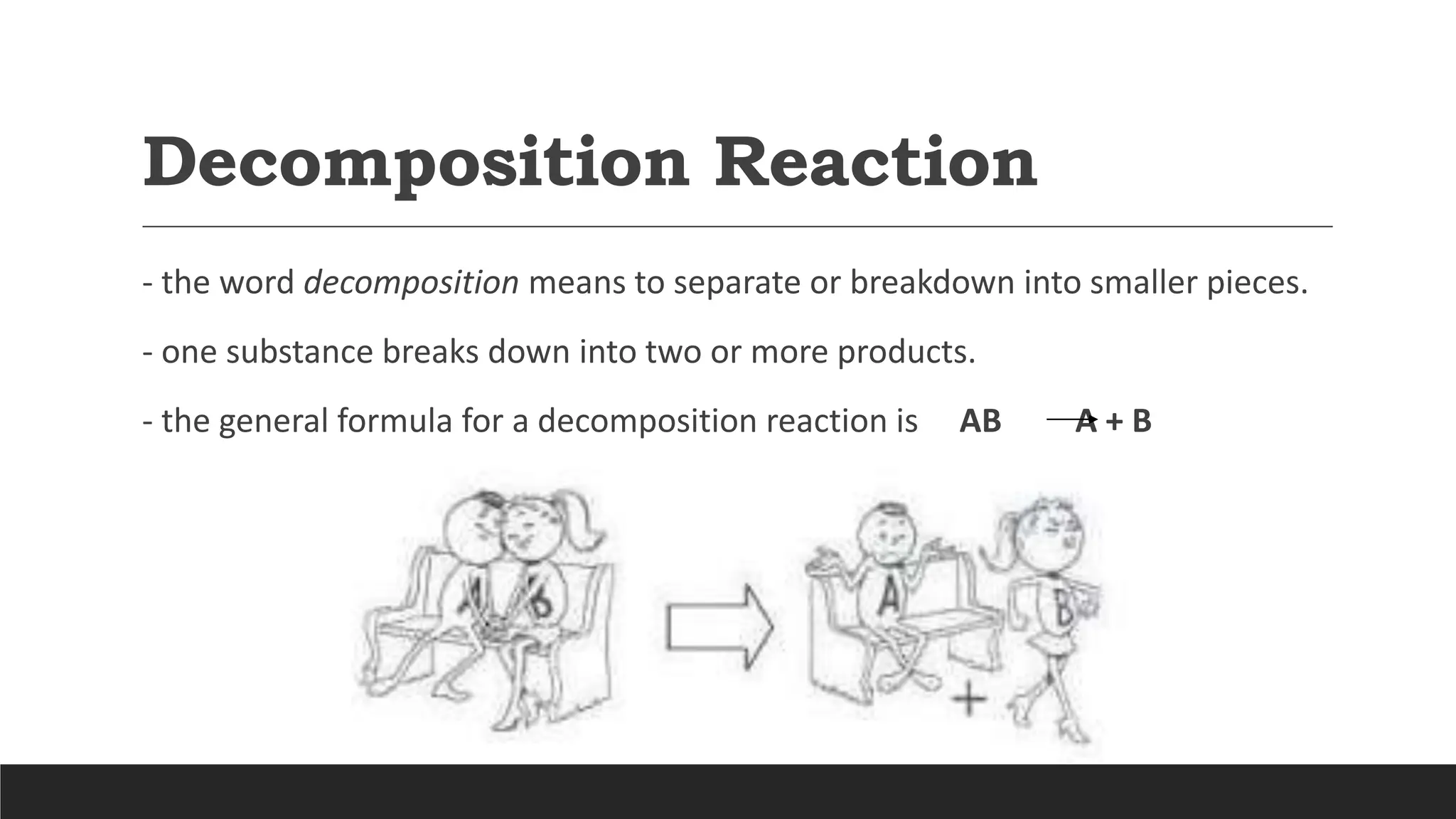
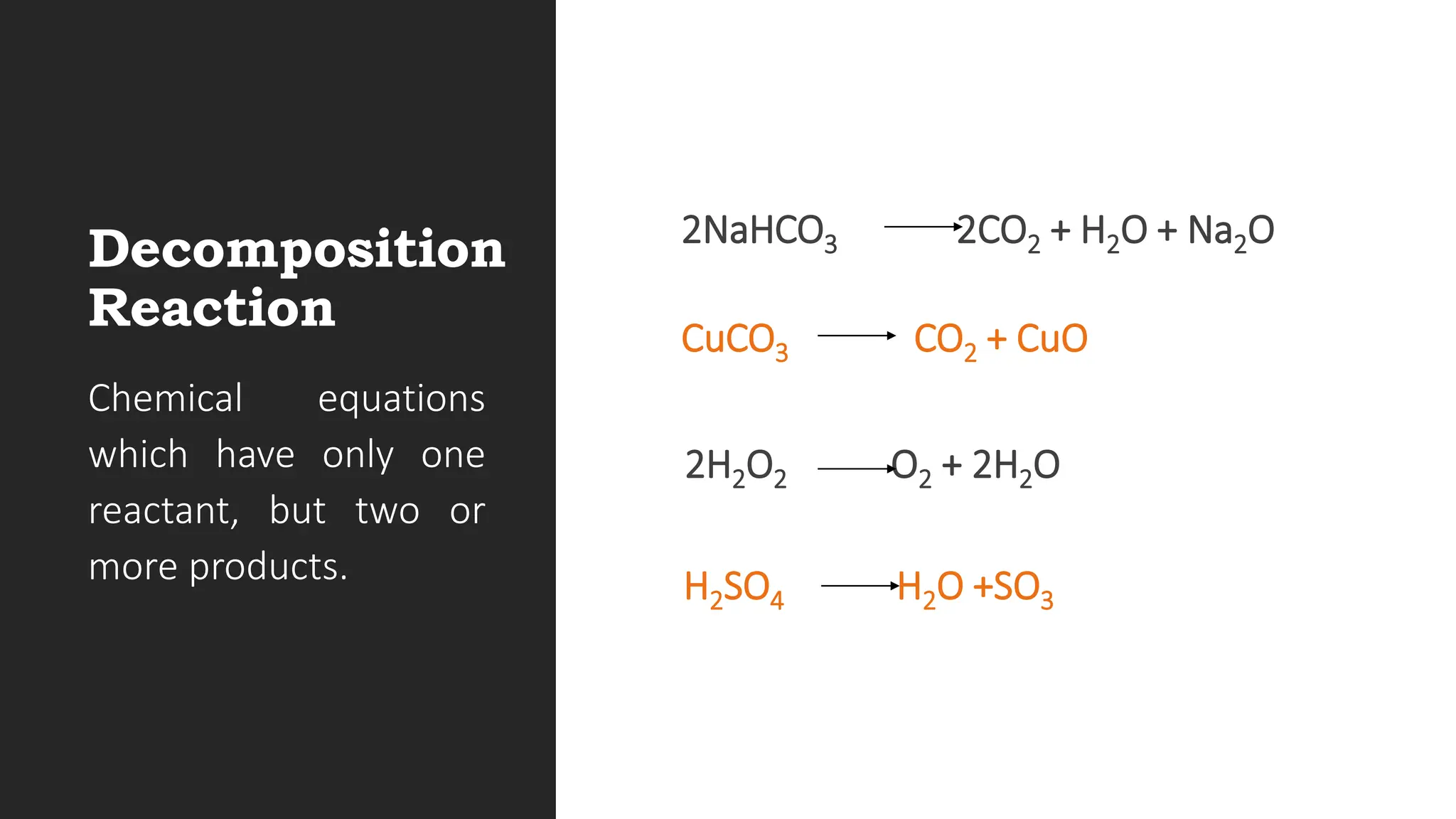
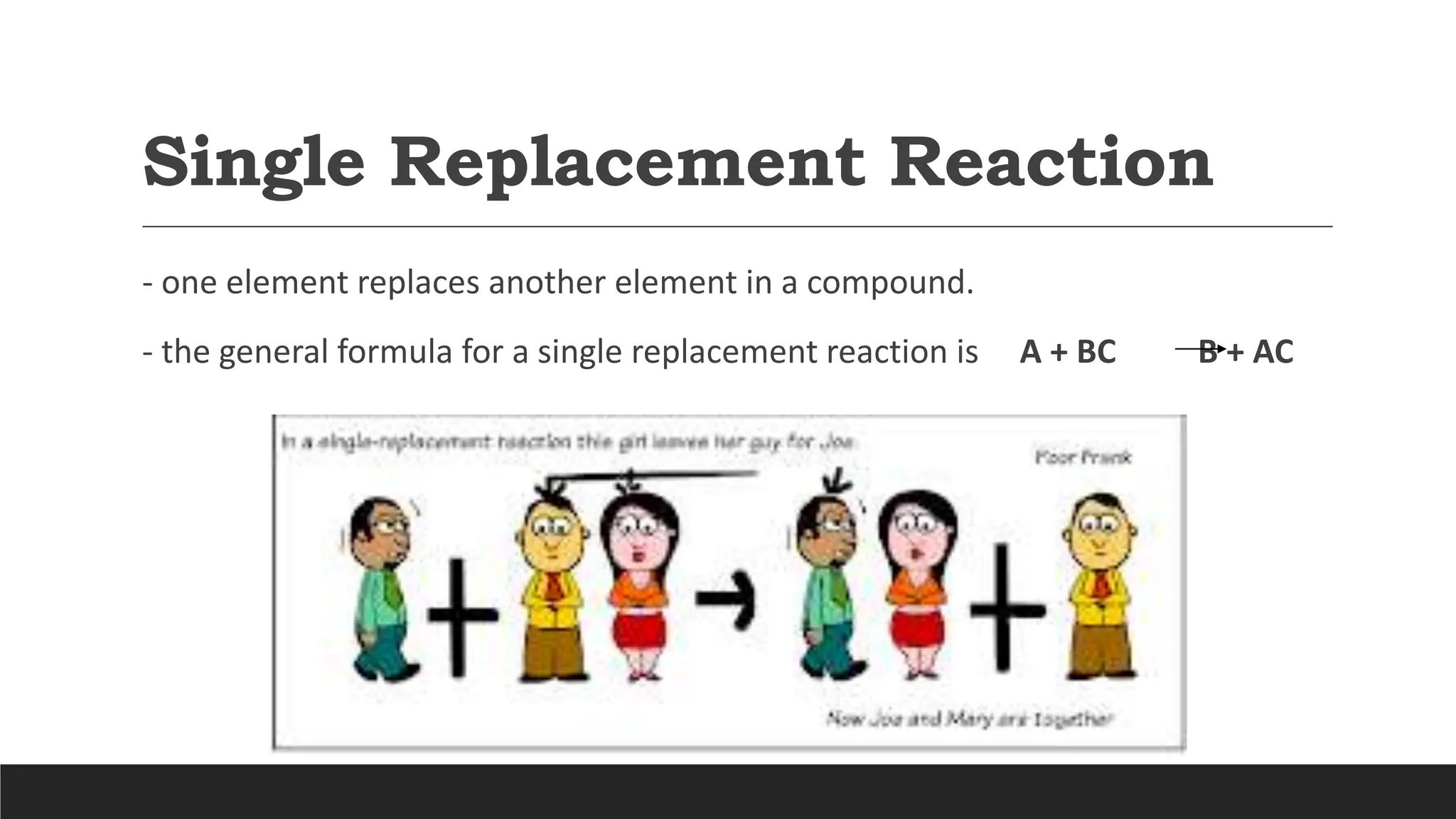
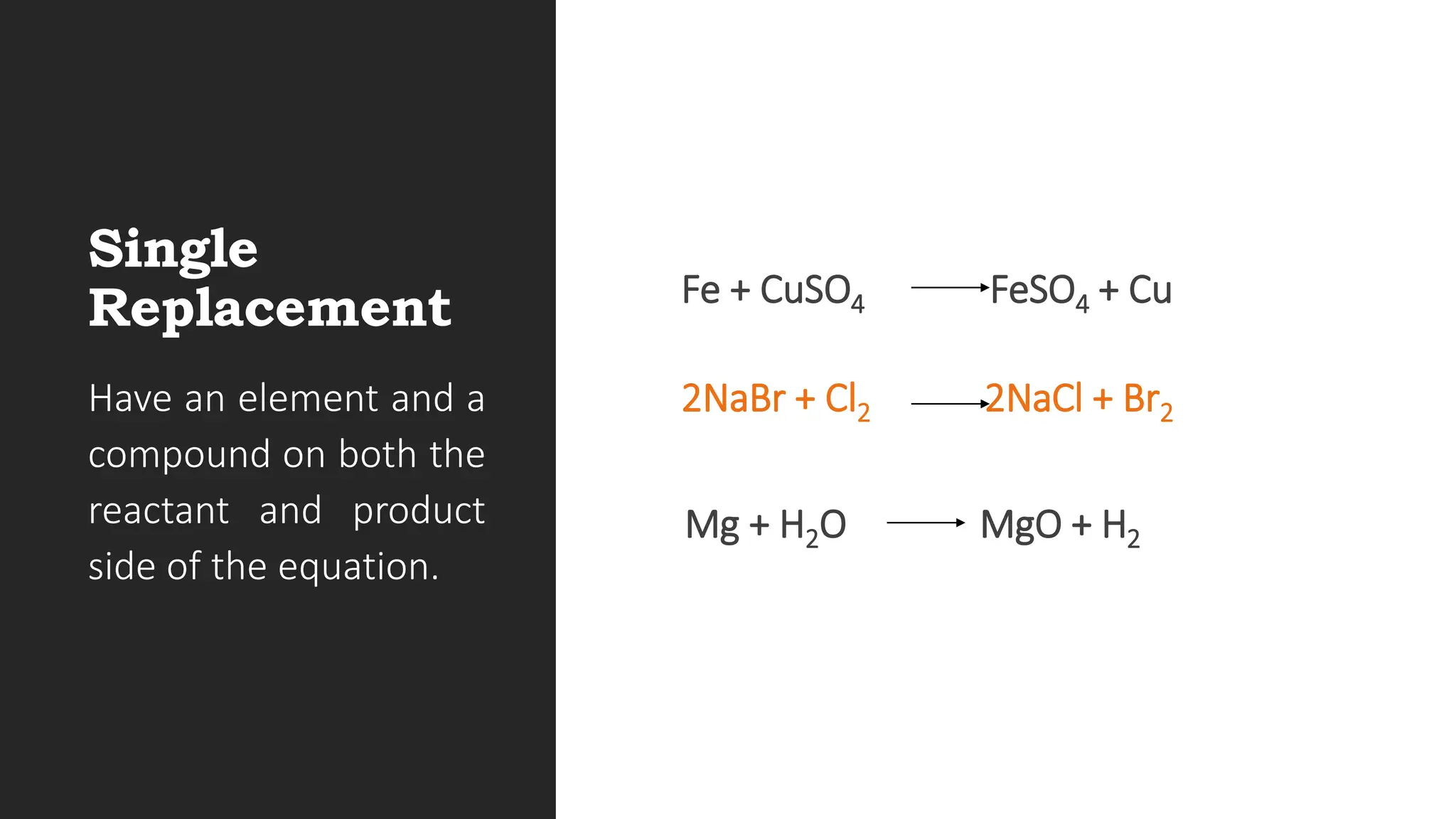
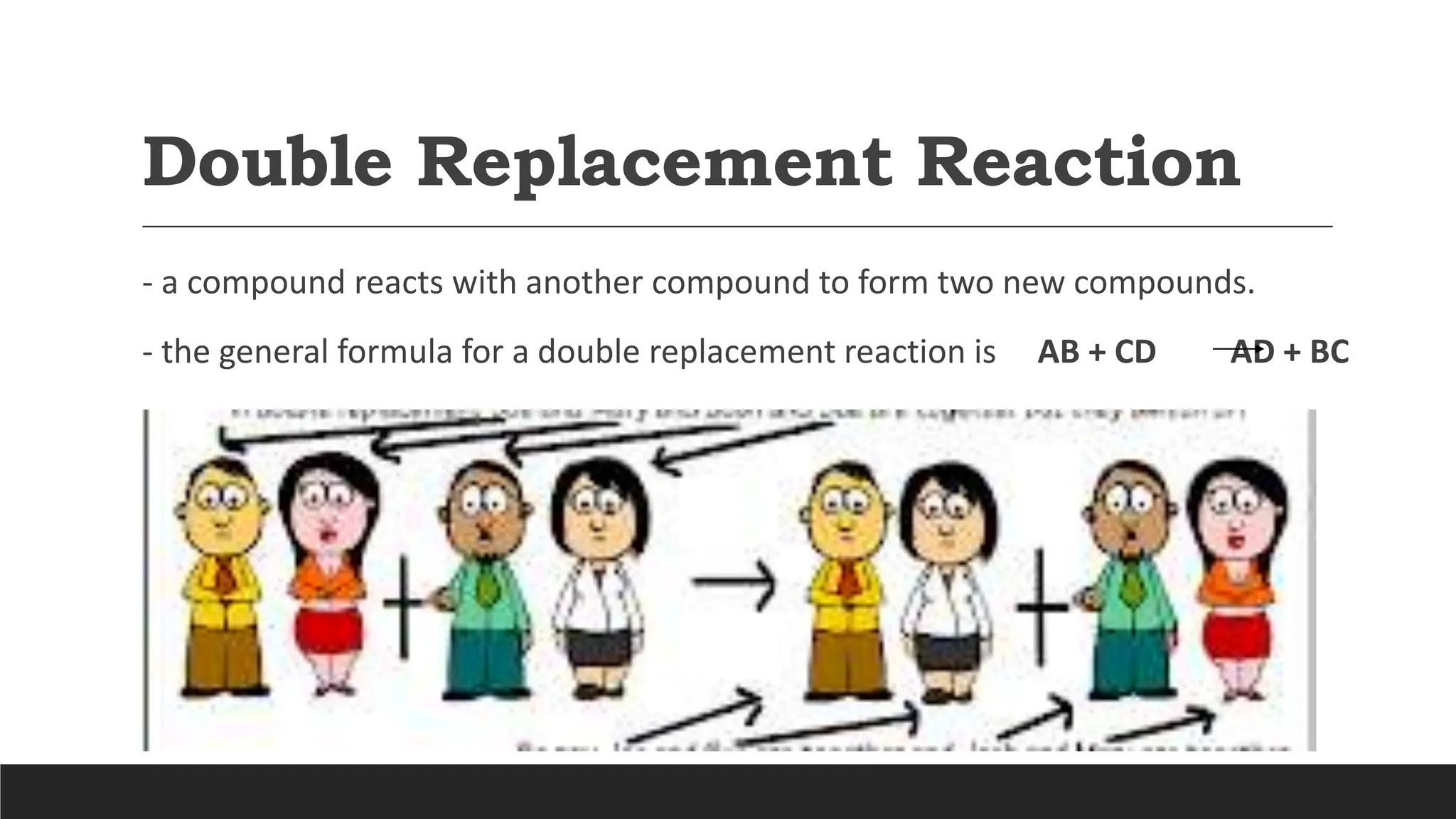
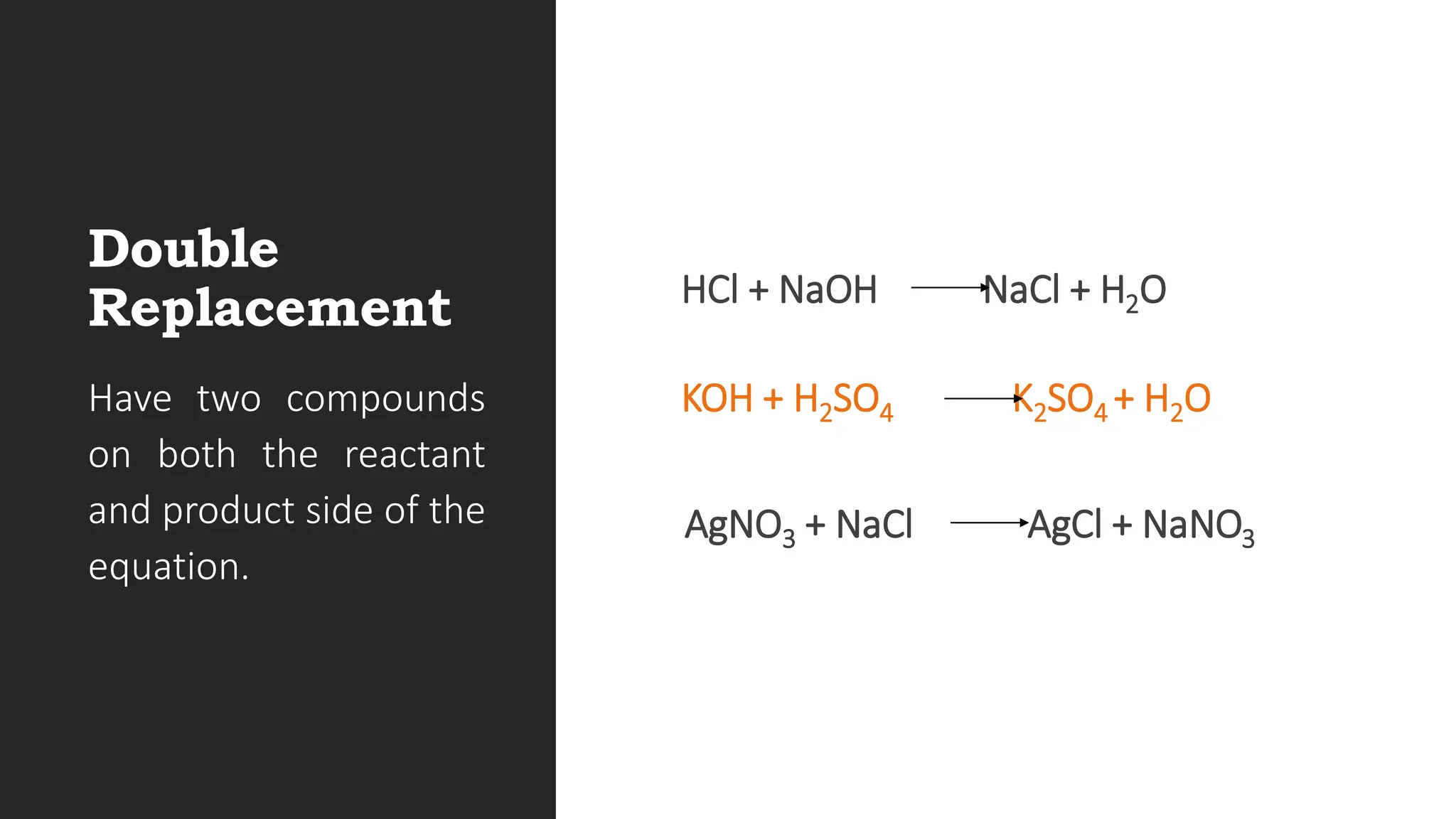
The document explains the basics of chemical reactions, defining them as processes where reactants are transformed into products. It details different types of reactions, including combination, decomposition, single replacement, and double replacement, along with their general formulas. The importance of effective particle collisions and activation energy is also emphasized for reactions to occur.