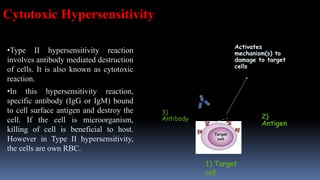
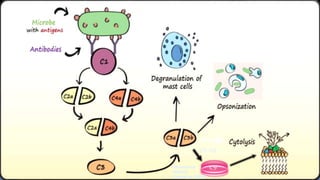
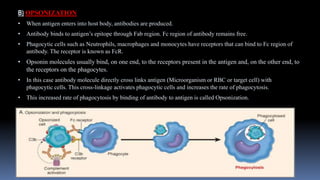
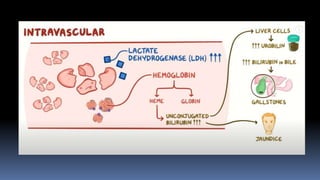
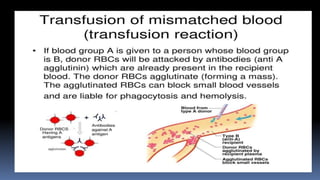
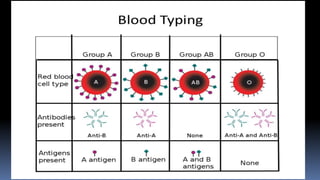
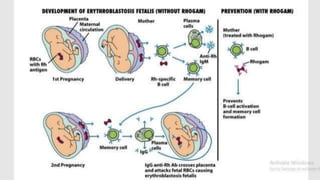
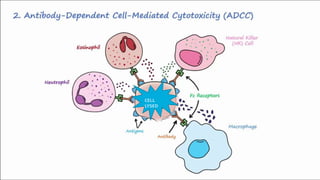
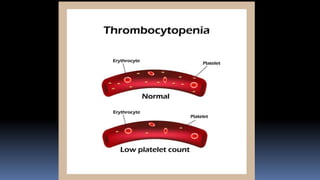
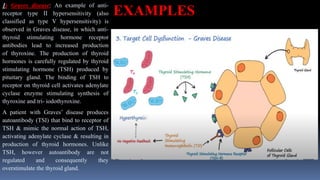
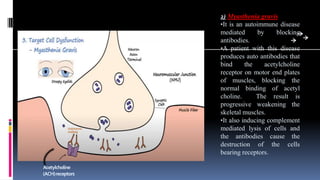
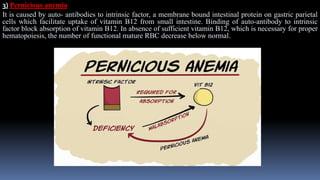
Type II hypersensitivity reactions involve antibody-mediated destruction of cells through three main mechanisms: complement-mediated cell lysis, antibody-dependent cell-mediated cytotoxicity (ADCC), and target cell dysfunction. Complement-mediated lysis results from membrane attack complex formation following complement activation by antigen-antibody complexes. ADCC occurs when antibodies bind to cell surfaces and recruit natural killer cells to induce apoptosis. Target cell dysfunction involves antibodies altering cell surface receptors and physiology rather than directly killing cells, as seen in Graves' disease and myasthenia gravis. Examples provided include autoimmune hemolytic anemia, hemolytic disease of the newborn, Goodpasture syndrome, and drug-induced thrombocytopenia.