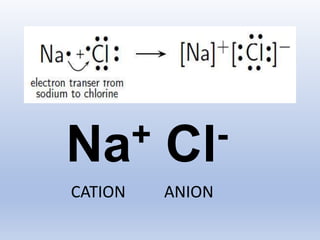
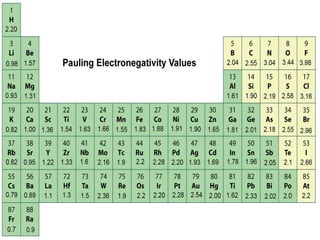
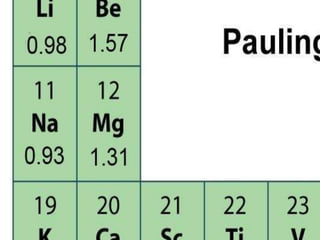
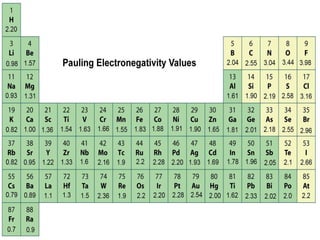
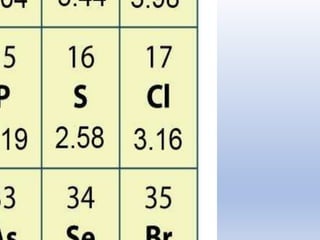
The document discusses the transfer of electrons during chemical bonding and ionic bonding specifically. It begins with an introduction to Lewis symbols and their use in representing valence electrons. It then explains how ionic bonding occurs through the transfer of electrons from a metal to a nonmetal to form ions, using sodium and chlorine as an example where sodium loses an electron to become Na+ and chlorine gains an electron to become Cl-. It further defines ionic bonding as the electrostatic attraction between oppositely charged ions that forms when there is a large difference in electronegativity between the atoms.