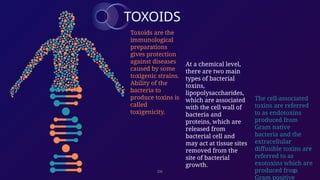
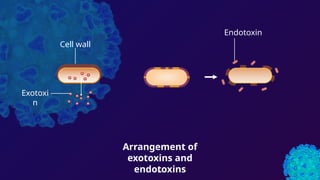
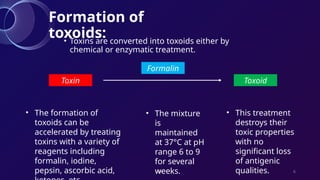
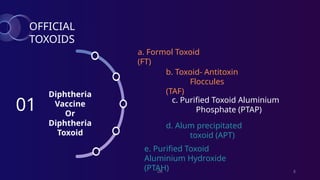
The document discusses toxoids, which are immunological preparations for protection against diseases from toxigenic bacteria. It distinguishes between exotoxins and endotoxins regarding their properties, sources, and methods of action. Additionally, it details the formation, purification, and examples of various official toxoids used in vaccines.