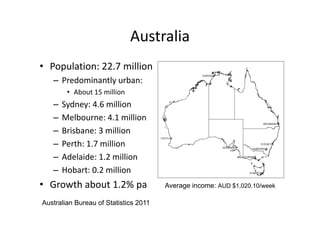
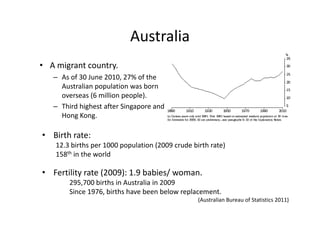
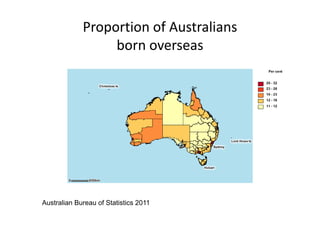
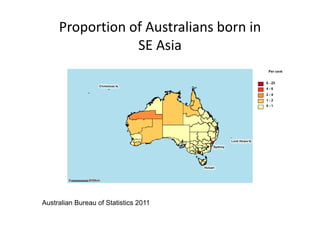
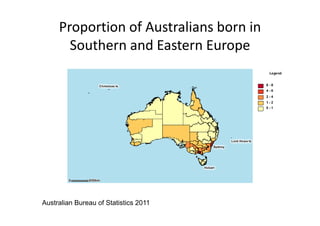
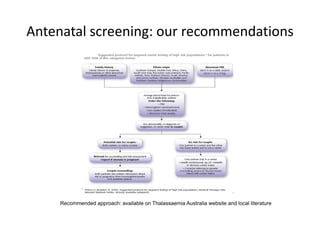
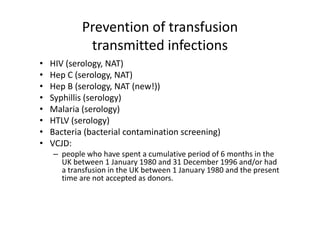
Thalassaemia is present among Australia's ethnically diverse population. There is no national registry or standardized antenatal screening policy. Estimates indicate around 326 patients with beta thalassaemia major nationally, though numbers may be higher without a registry. Diagnostic testing and genetic counseling are available through specialist centers and hospital laboratories nationwide.