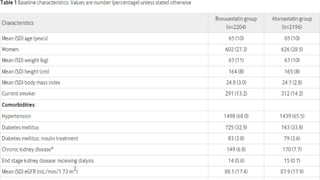
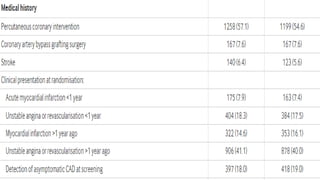
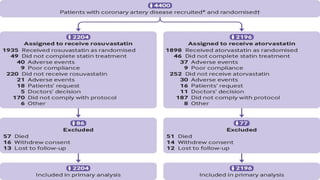
The document provides an overview of statins, their efficacy in treating coronary artery disease, and a three-year randomized trial comparing rosuvastatin and atorvastatin. It highlights no significant difference in primary outcomes between the two drugs but notes a higher incidence of new onset diabetes and cataract surgery in the rosuvastatin group. The study emphasizes the importance of monitoring side effects and tailoring statin choice to patient profiles.