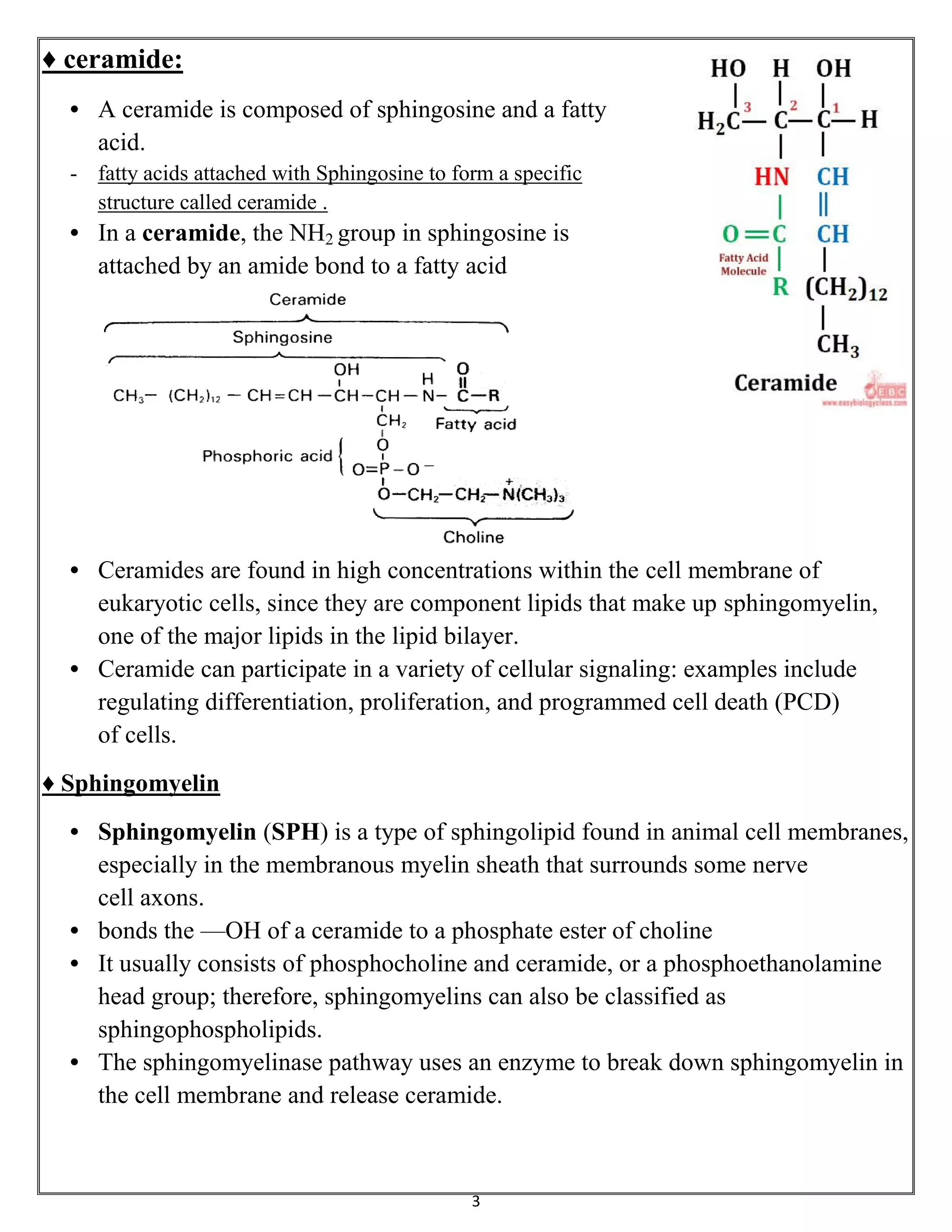
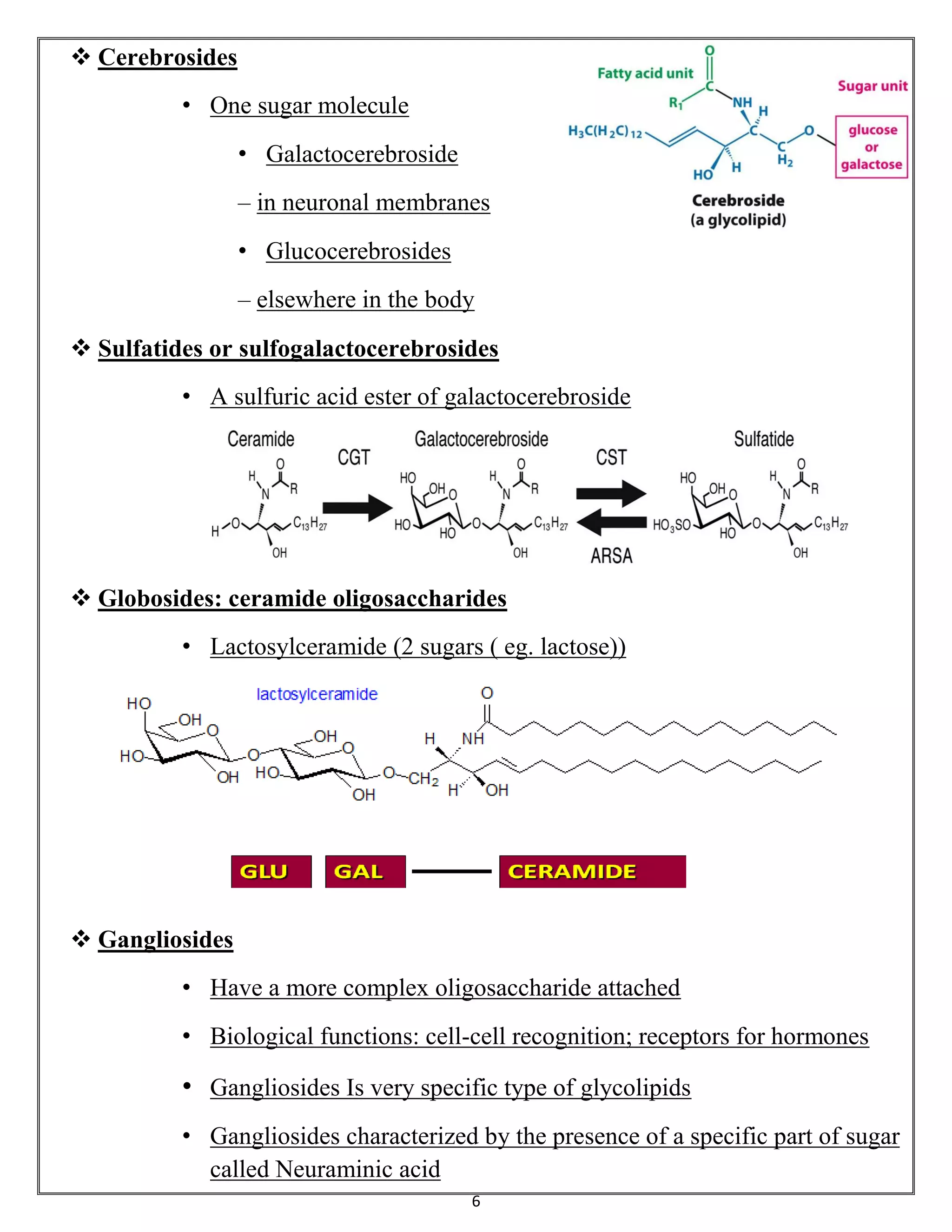
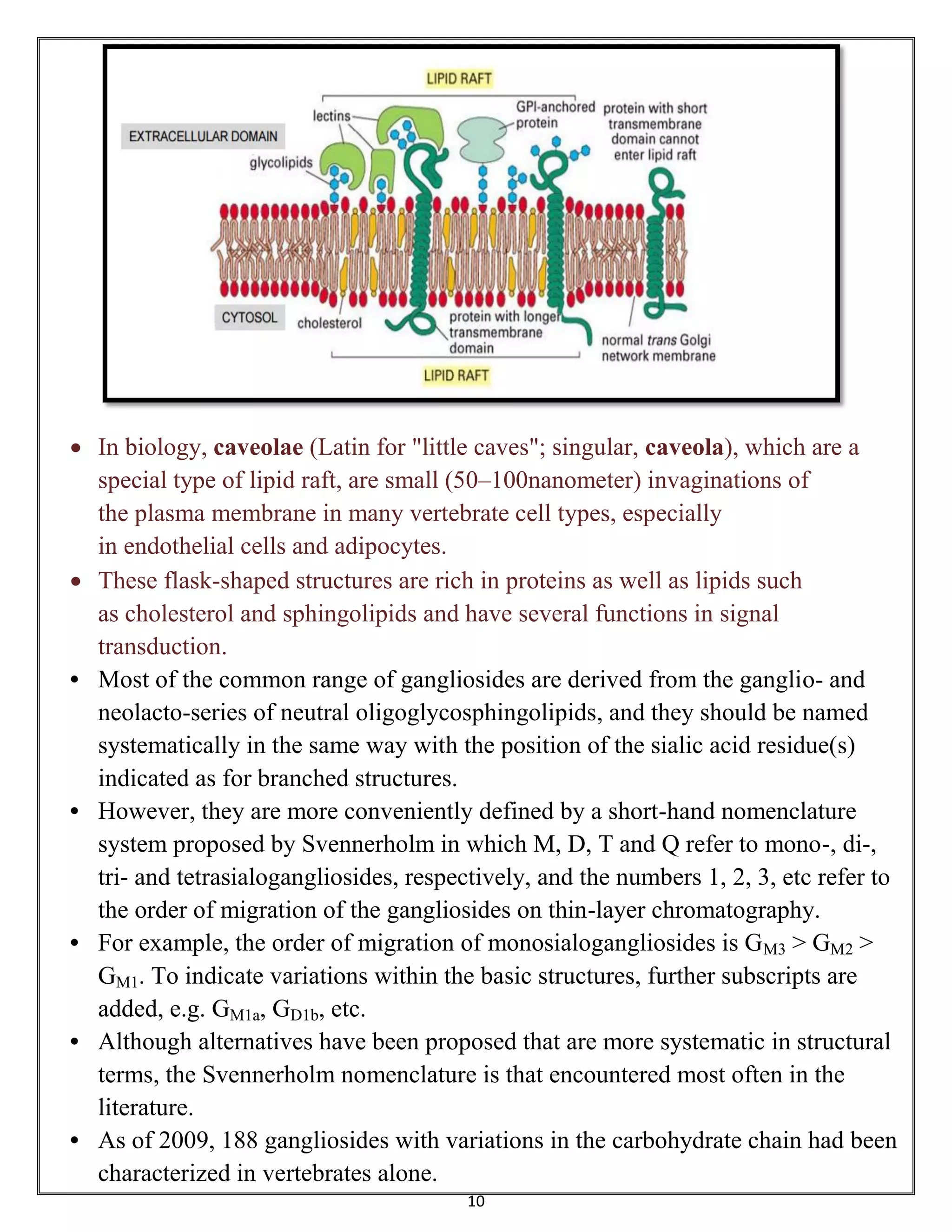
Sphingolipids are a class of lipids important for cell membranes and signaling, with sphingosine as a key component. They include various forms such as ceramides, sphingomyelin, and glycolipids like cerebrosides and gangliosides, which play roles in cell recognition and signaling. Gangliosides, in particular, are complex glycolipids found in neuronal membranes, crucial for neuronal development and functions.