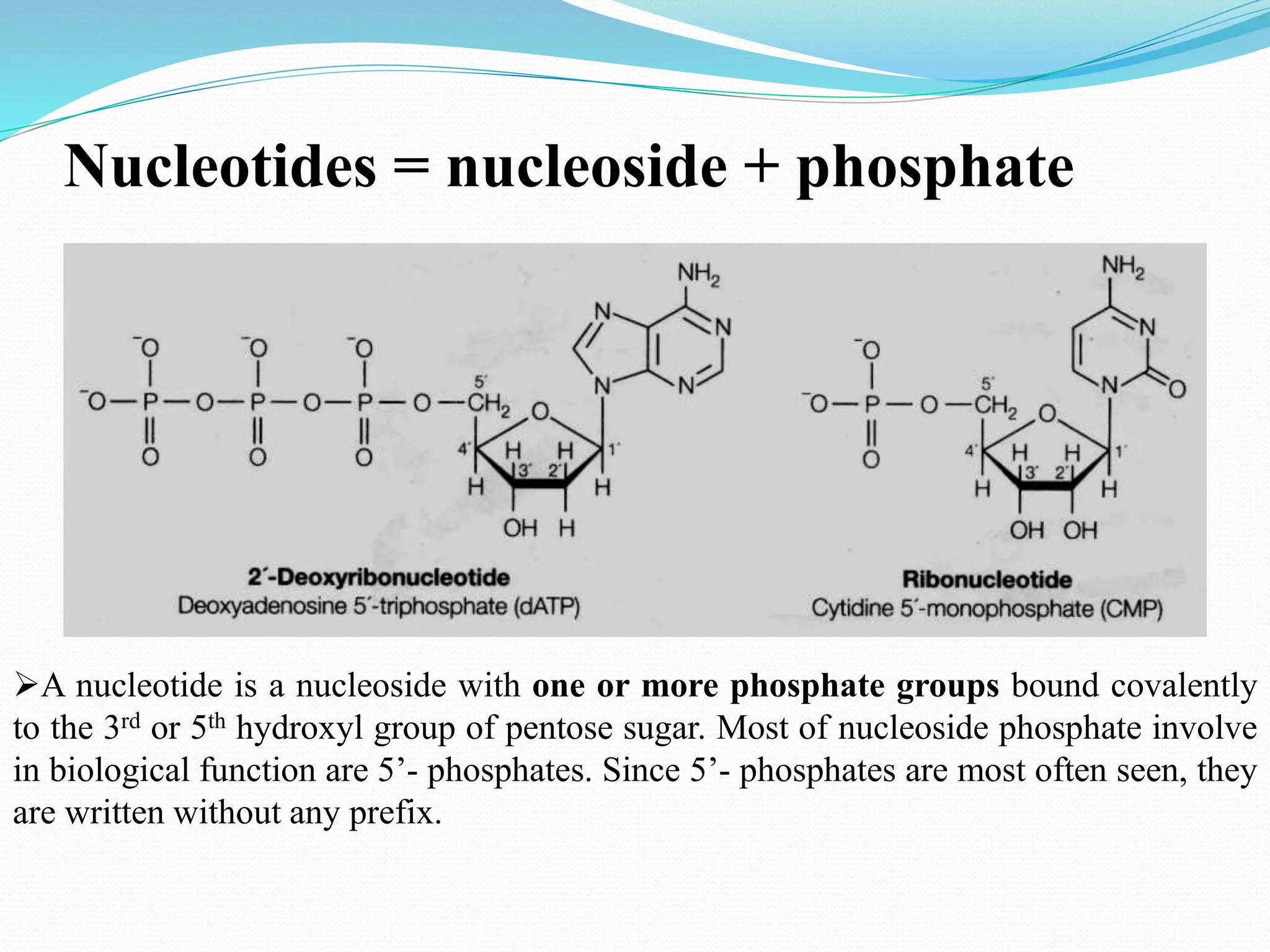
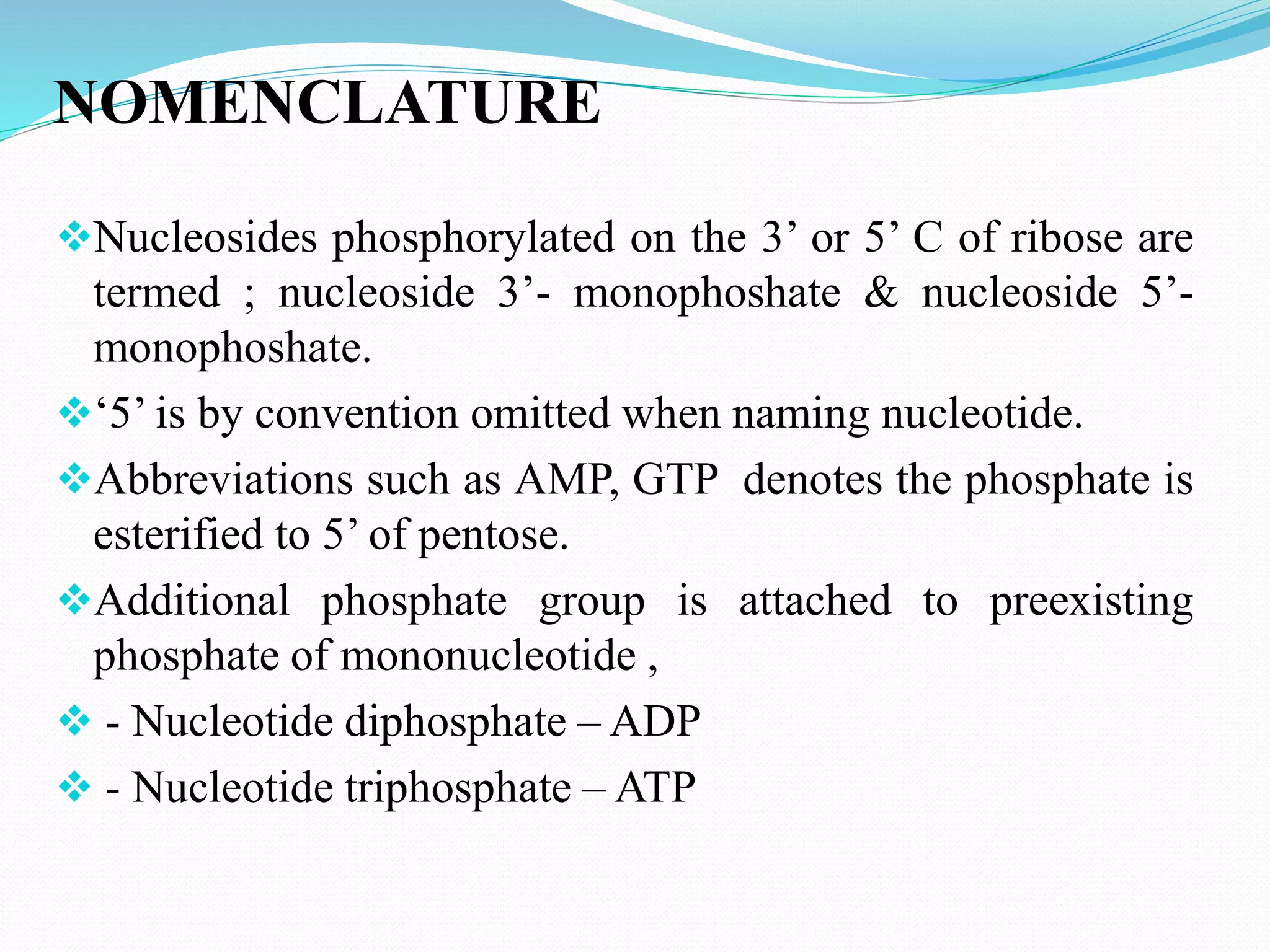
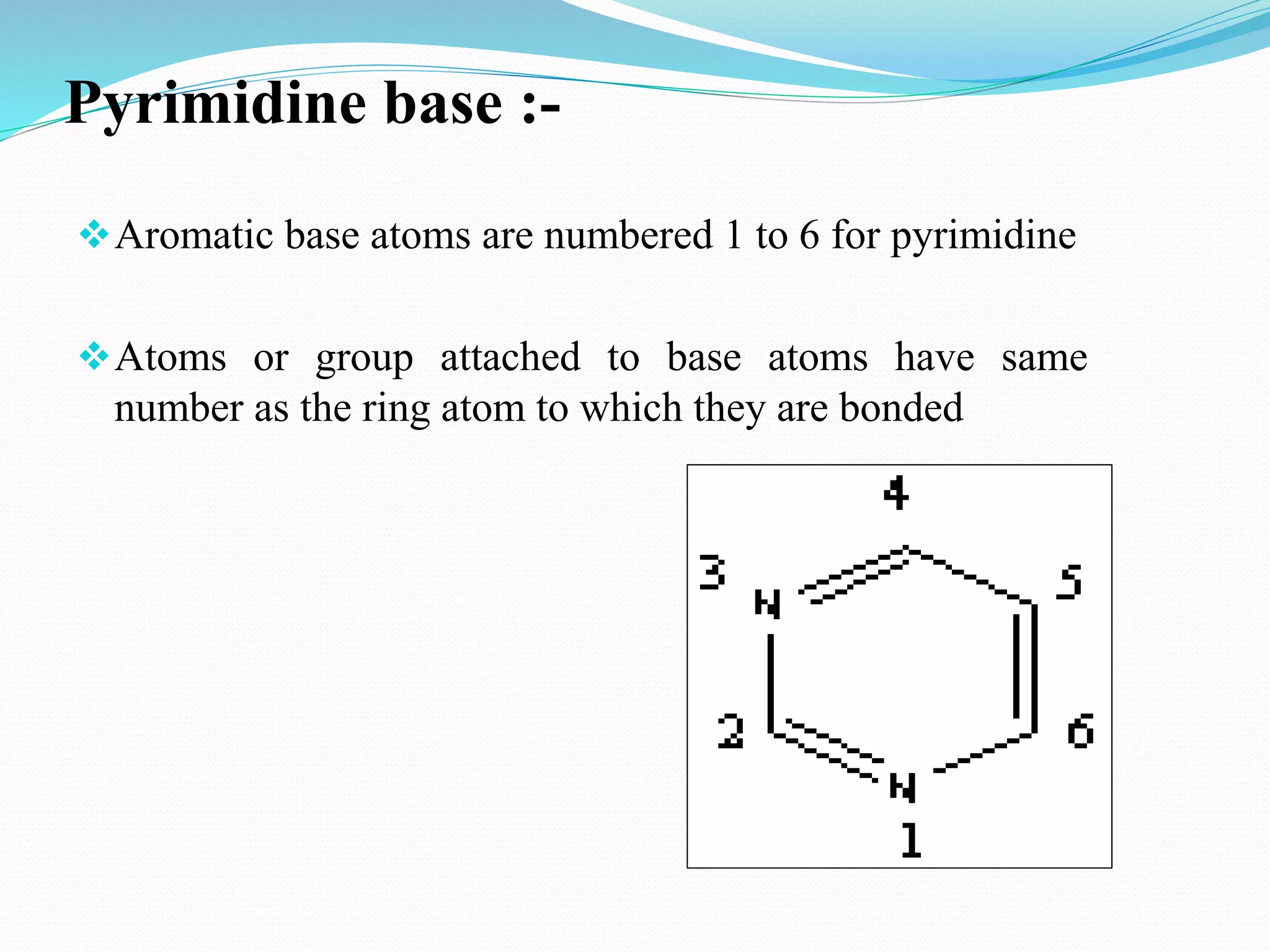
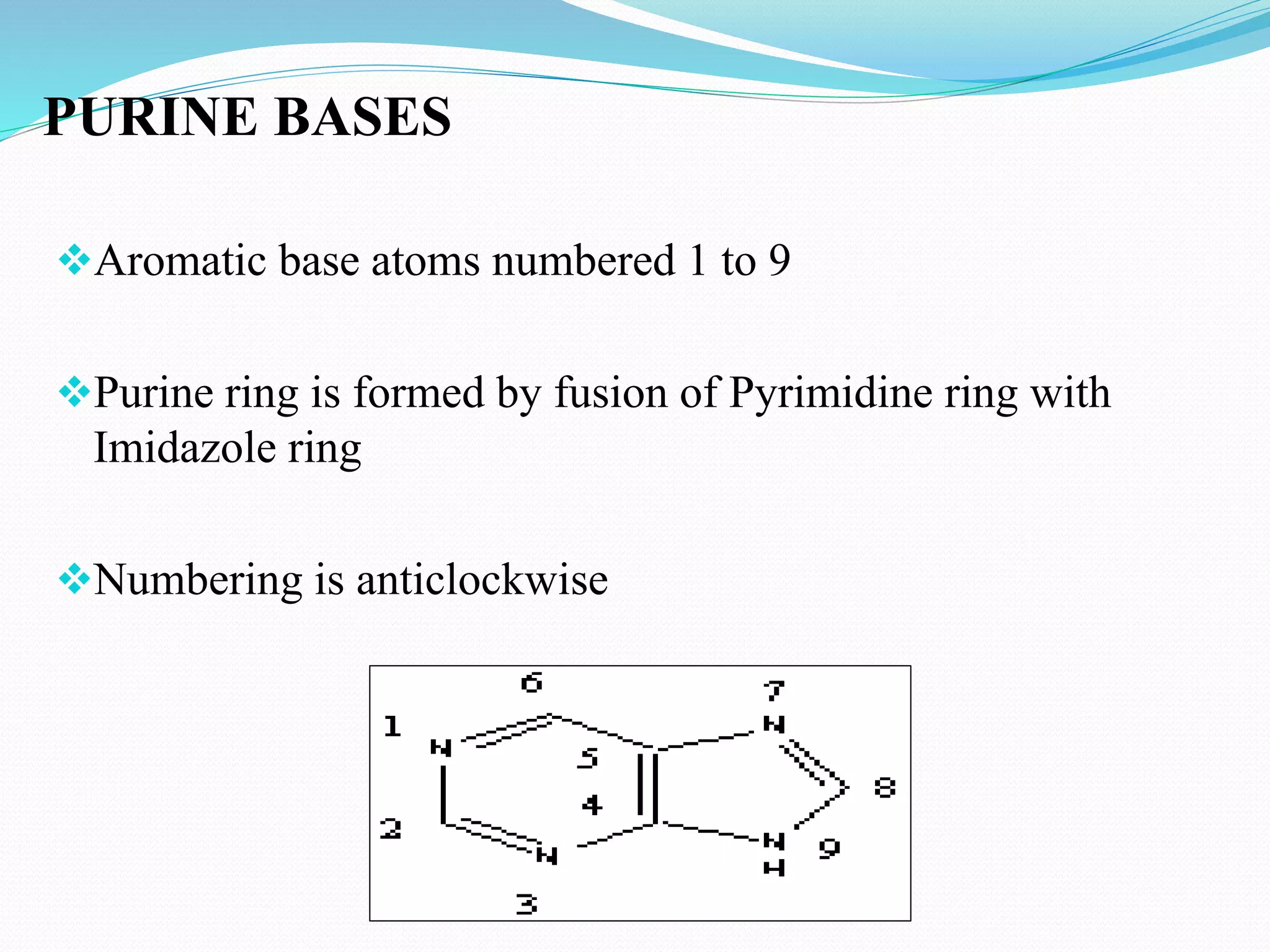
This document discusses nucleotide chemistry. Nucleotides are organic compounds composed of a phosphate group, nitrogenous base, and a sugar molecule. They serve as the building blocks of nucleic acids DNA and RNA. Nucleotides also function as sources of chemical energy through molecules like ATP and GTP, and participate in cellular signaling through molecules like cAMP and cGMP. The document goes on to describe the specific sugars, bases, nucleosides, and nucleotides that make up DNA and RNA. It provides details on nucleotide nomenclature and classification and discusses important adenosine-containing nucleotides and their roles, such as ATP serving as an important energy source in many cellular processes.