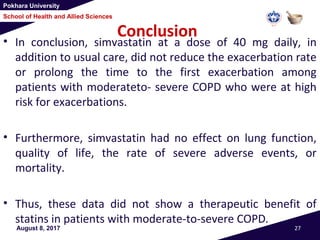
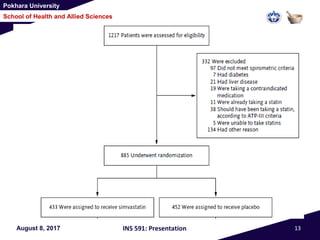
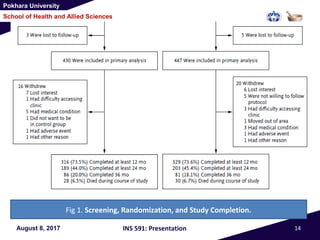
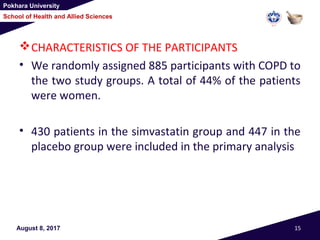
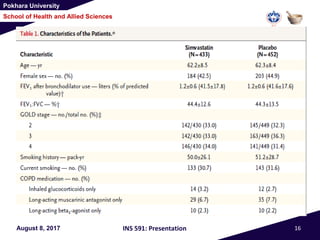
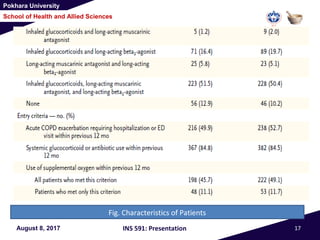
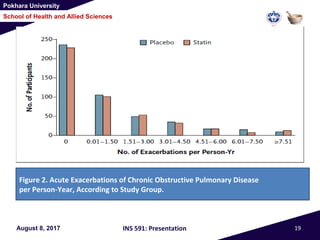
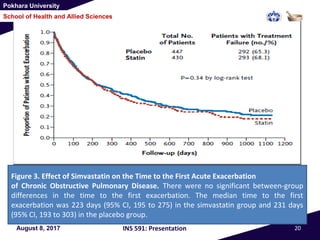
This document summarizes a study that investigated whether simvastatin could prevent exacerbations in patients with moderate to severe COPD. The study involved 885 patients randomized to receive either simvastatin 40mg daily or a placebo. The primary outcome was the exacerbation rate, and secondary outcomes included time to first exacerbation, lung function, quality of life, and adverse events. The results showed no significant differences between the simvastatin and placebo groups in exacerbation rate, time to first exacerbation, or secondary outcomes. Simvastatin 40mg daily did not demonstrate a therapeutic benefit for reducing exacerbations in patients with moderate to severe COPD.







![Pokhara University
School of Health and Allied Sciences
Study visits
• Participants were followed at clinic visits every
months and by means of monthly telephone
contacts.
• Spirometric variable and quality of life assessed with
the use of the St. George’s Respiratory Questionnaire
[SGRQ] and the Medical Outcomes Study 36-Item
Short-Form Health Survey [SF-36] were measured at
baseline and at 12,24, and 36 months.
August 8, 2017 9](https://image.slidesharecdn.com/seminar2anita-170808060158/85/Seminar-first-semester-Simvastation-in-COPD-9-320.jpg)





![Pokhara University
School of Health and Allied Sciences
ADVERSE EVENTS
• The most common adverse events, aside from
exacerbations, were pneumonia and other respiratory
and cardiovascular events.
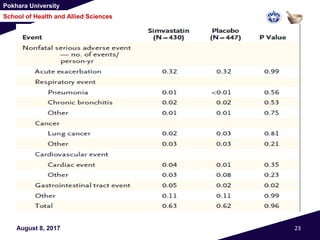
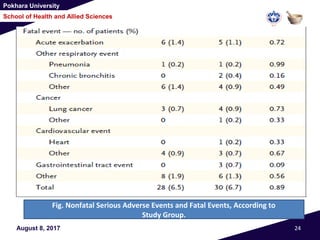
• Except for nonfatal serious adverse events involving
the gastrointestinal tract, which were more frequent
with simvastatin than with placebo (in 30 patients
[0.05 events per personyear] vs. 17 patients [0.02
events per person-year], P = 0.02) (Table 2)
• There were 28 deaths in the simvastatin group and 30
in the placebo group (P = 0.89).
August 8, 2017 22](https://image.slidesharecdn.com/seminar2anita-170808060158/85/Seminar-first-semester-Simvastation-in-COPD-22-320.jpg)