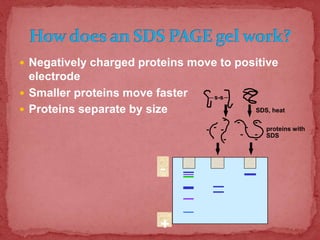
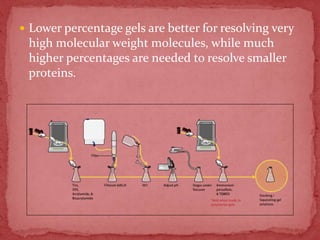
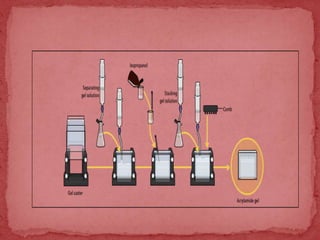
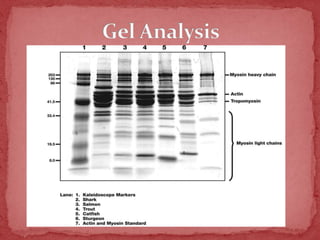
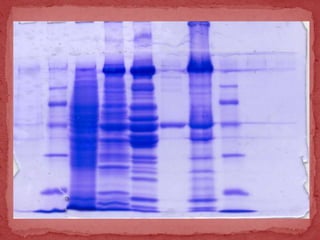
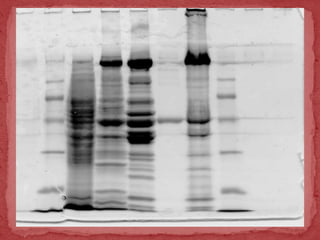
Sodium dodecylsulphate-polyacrylamide gel electrophoresis (SDS-PAGE) is a method used to separate proteins by size. It involves extracting proteins from a sample, solubilizing and denaturing the proteins using SDS detergent and heat, separating the proteins on a polyacrylamide gel based on their size and charge, and staining proteins for visualization and analysis. Smaller proteins move faster through the gel towards the positive electrode. SDS-PAGE allows determination of protein size, identification, purity, and quantification.