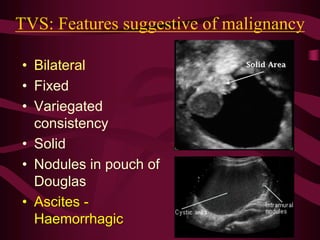
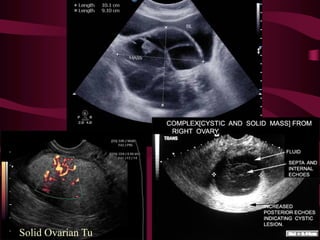
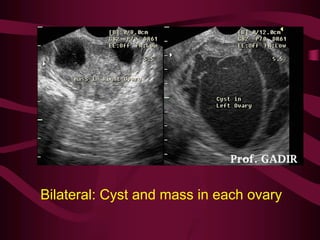
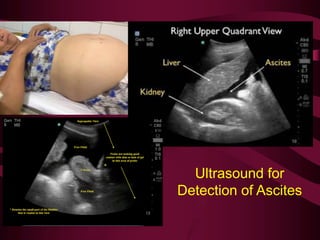
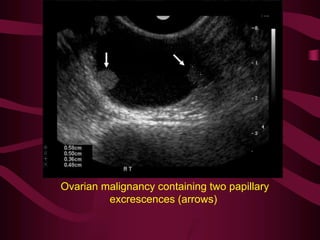
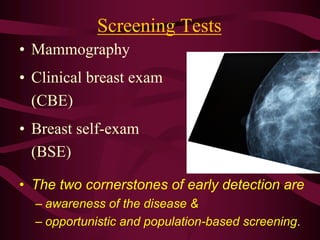
This document discusses screening guidelines for various cancers in women, including cervical, ovarian, endometrial, and breast cancer. It provides details on:
- The purpose and criteria for effective cancer screening programs
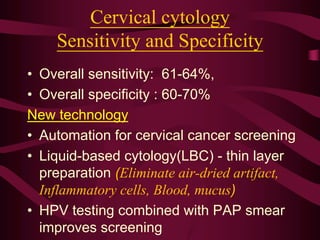
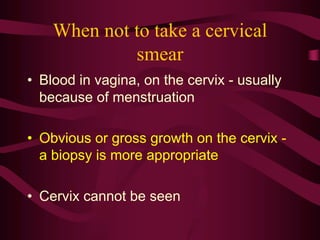
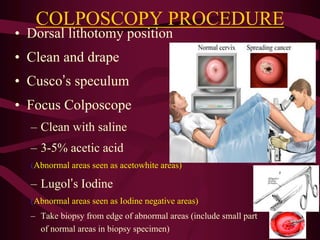
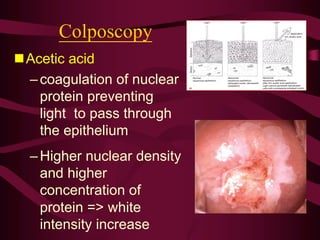
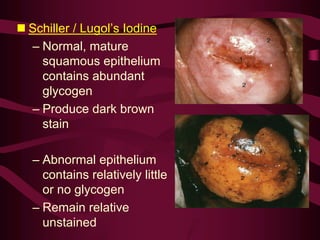
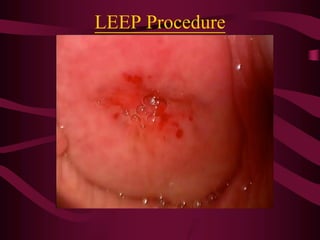
- Screening recommendations and techniques for each cancer type, including cervical cytology, colposcopy, transvaginal ultrasound, and mammography
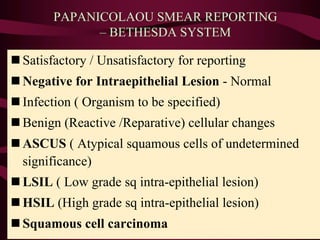
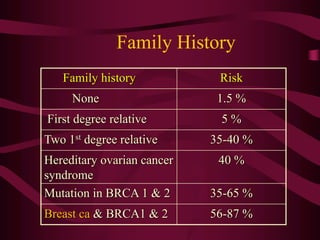
- Management of abnormal screening results, such as follow up tests or procedures for lesions of different grades
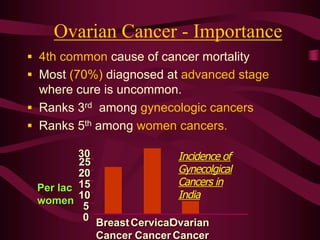
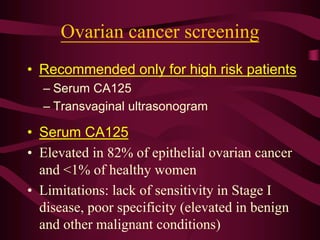
- Challenges with screening for some cancers like ovarian cancer due to limitations in current screening tests