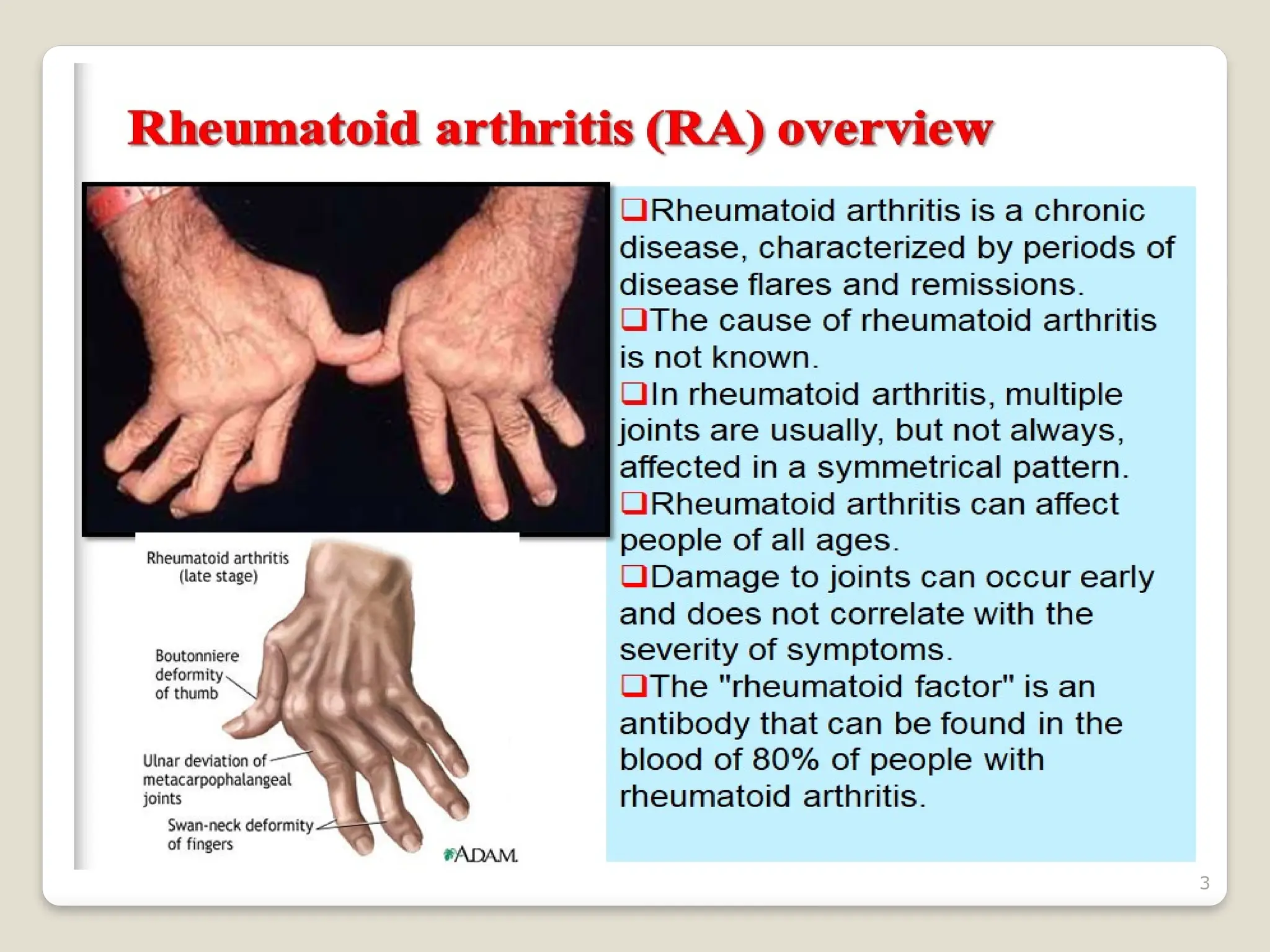
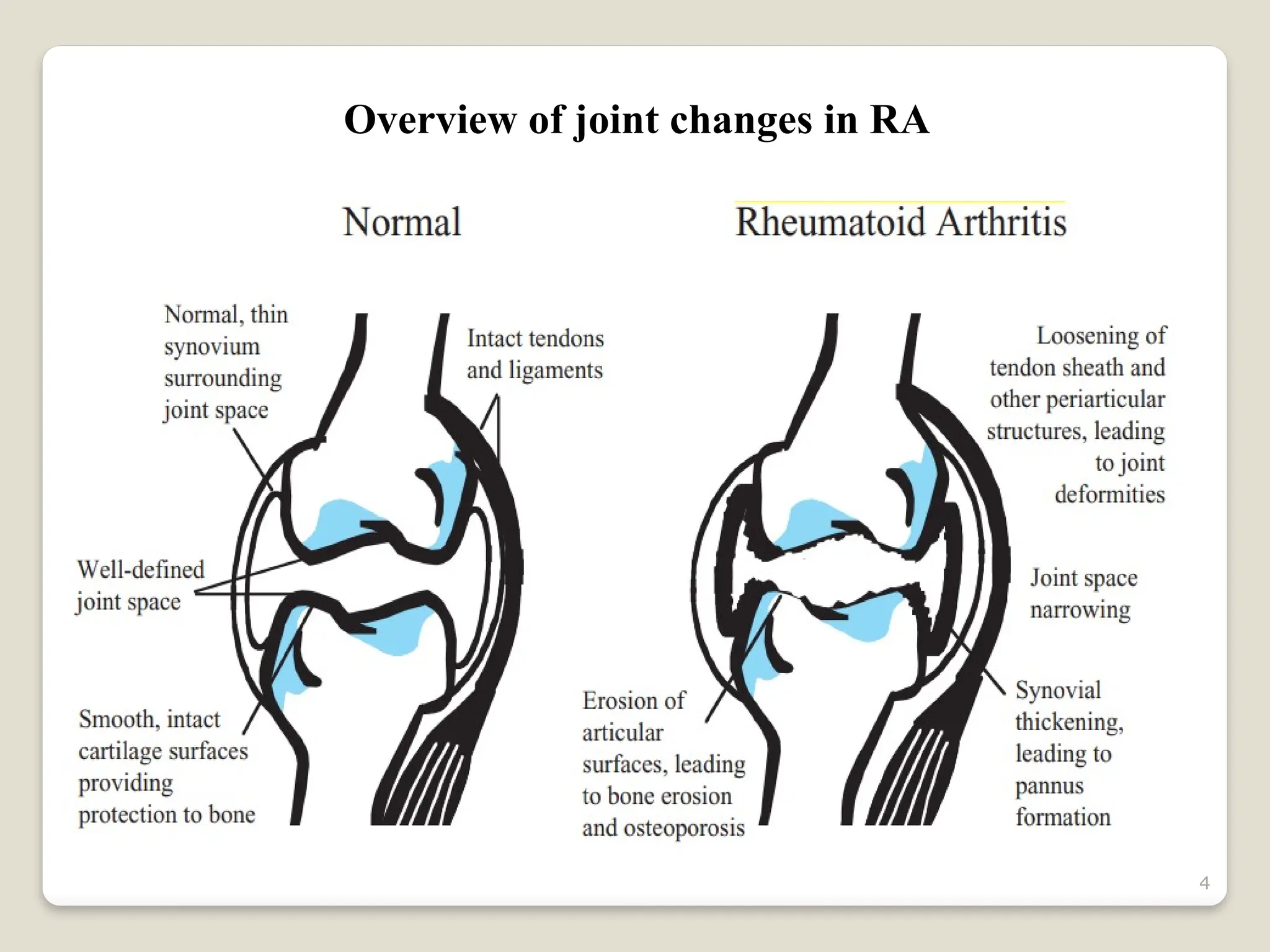
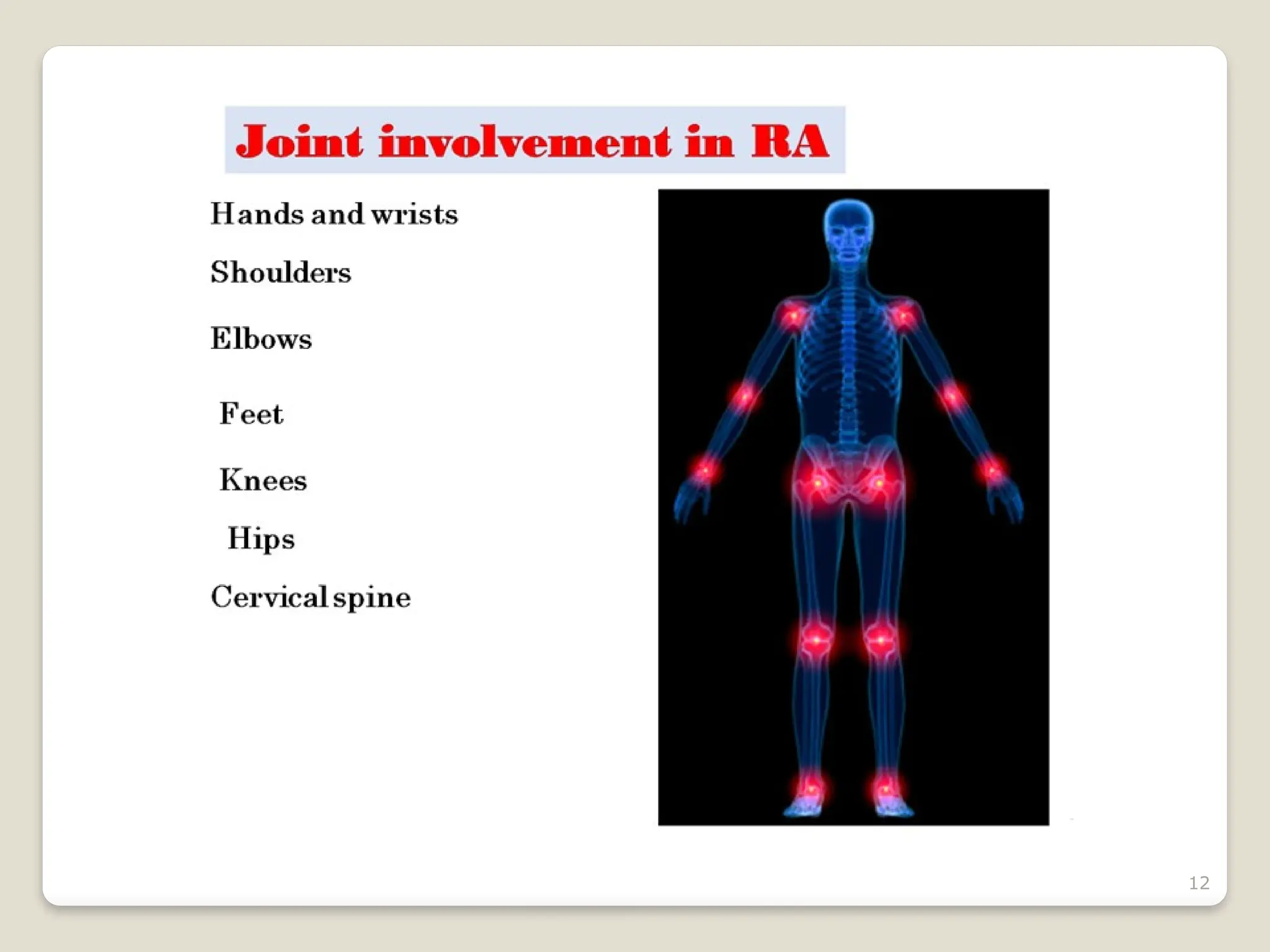
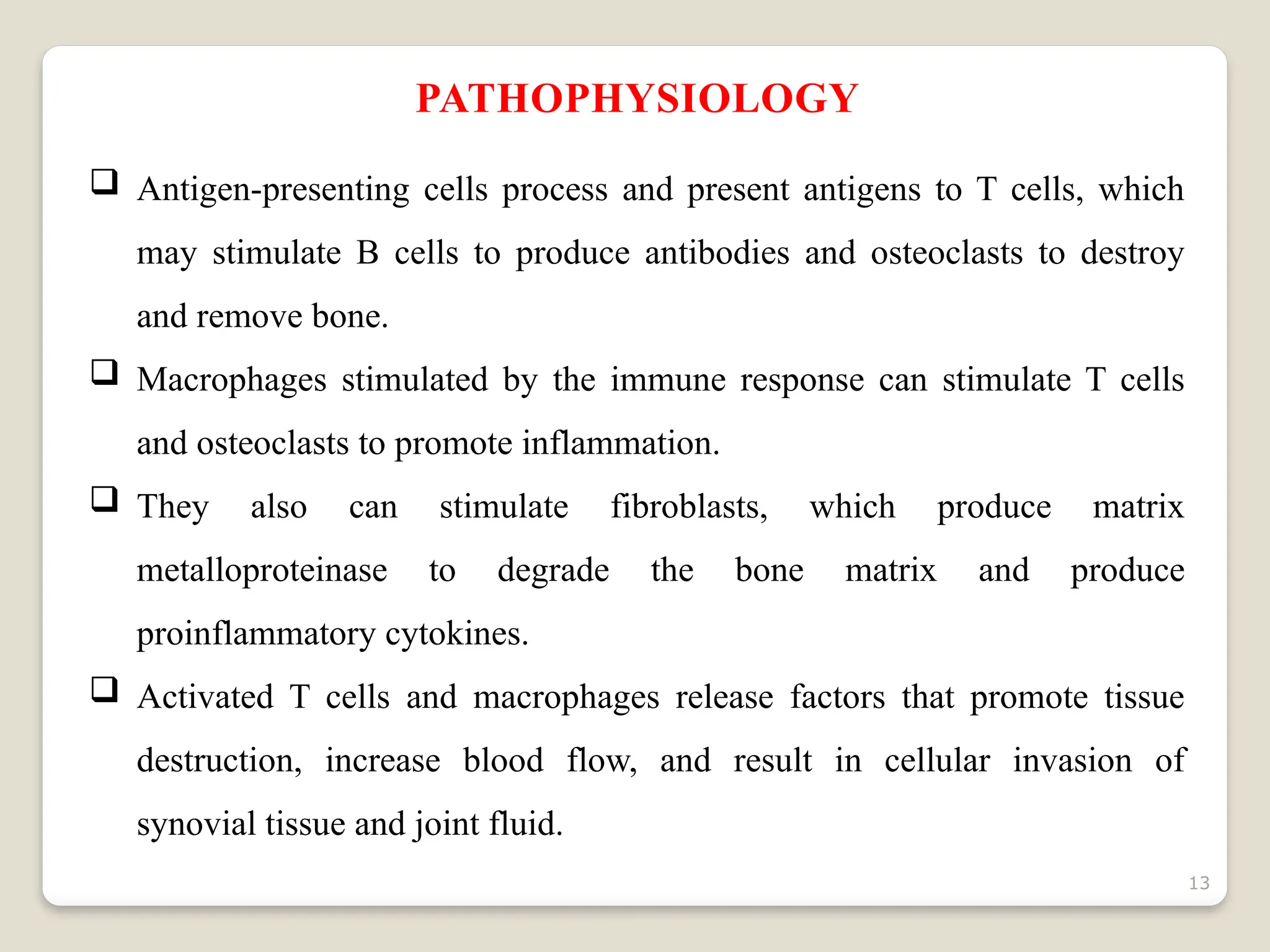
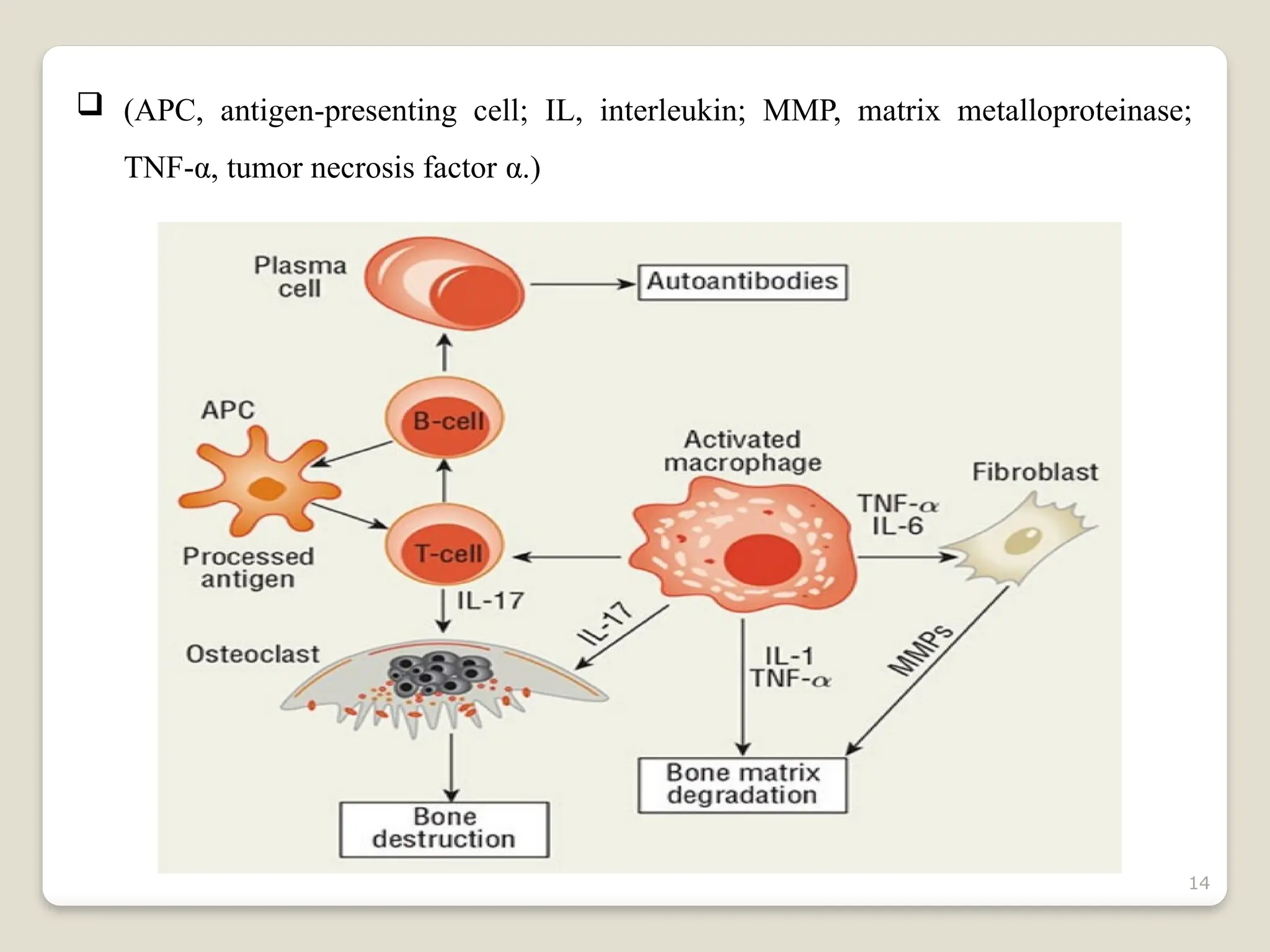
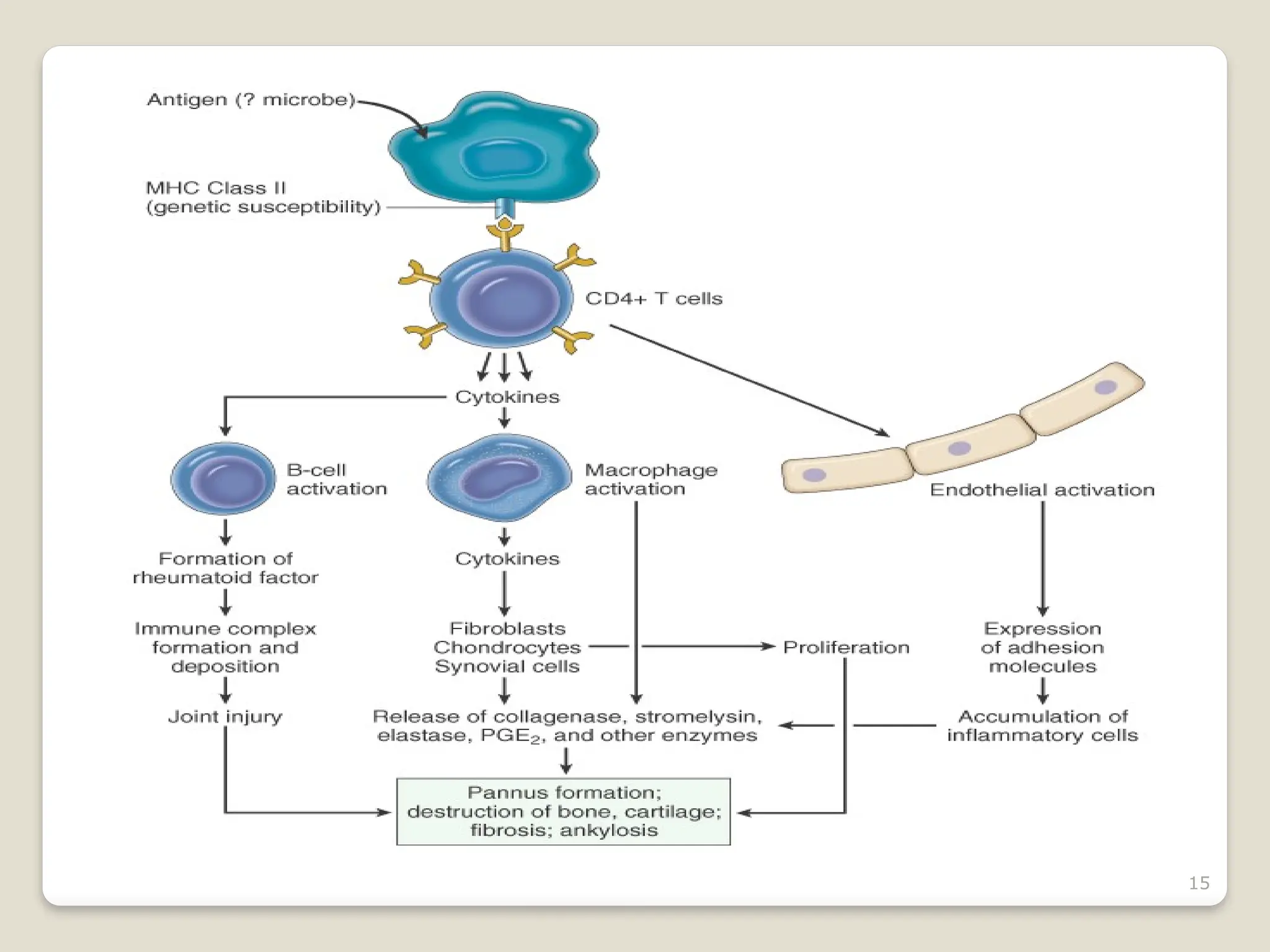
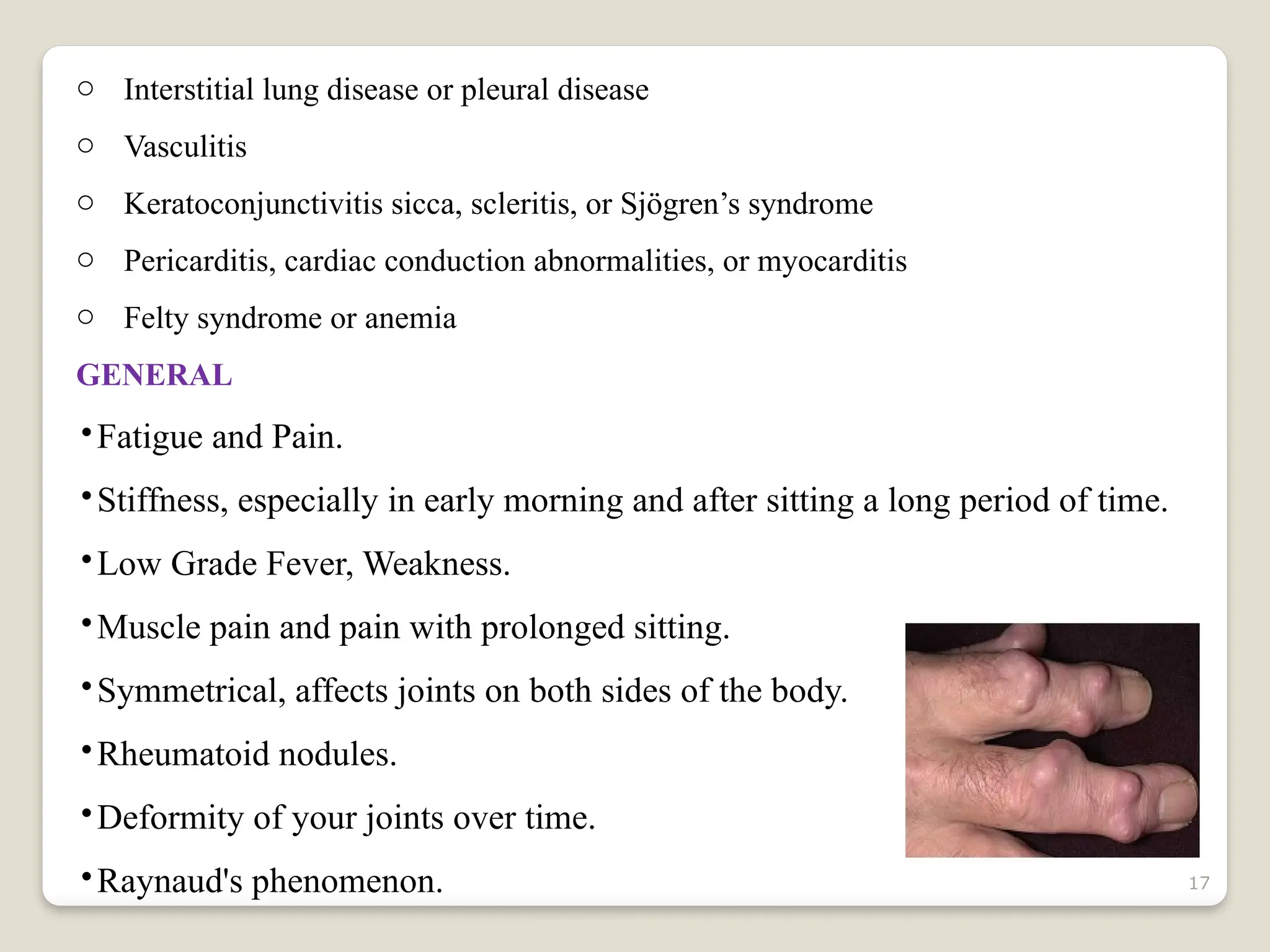
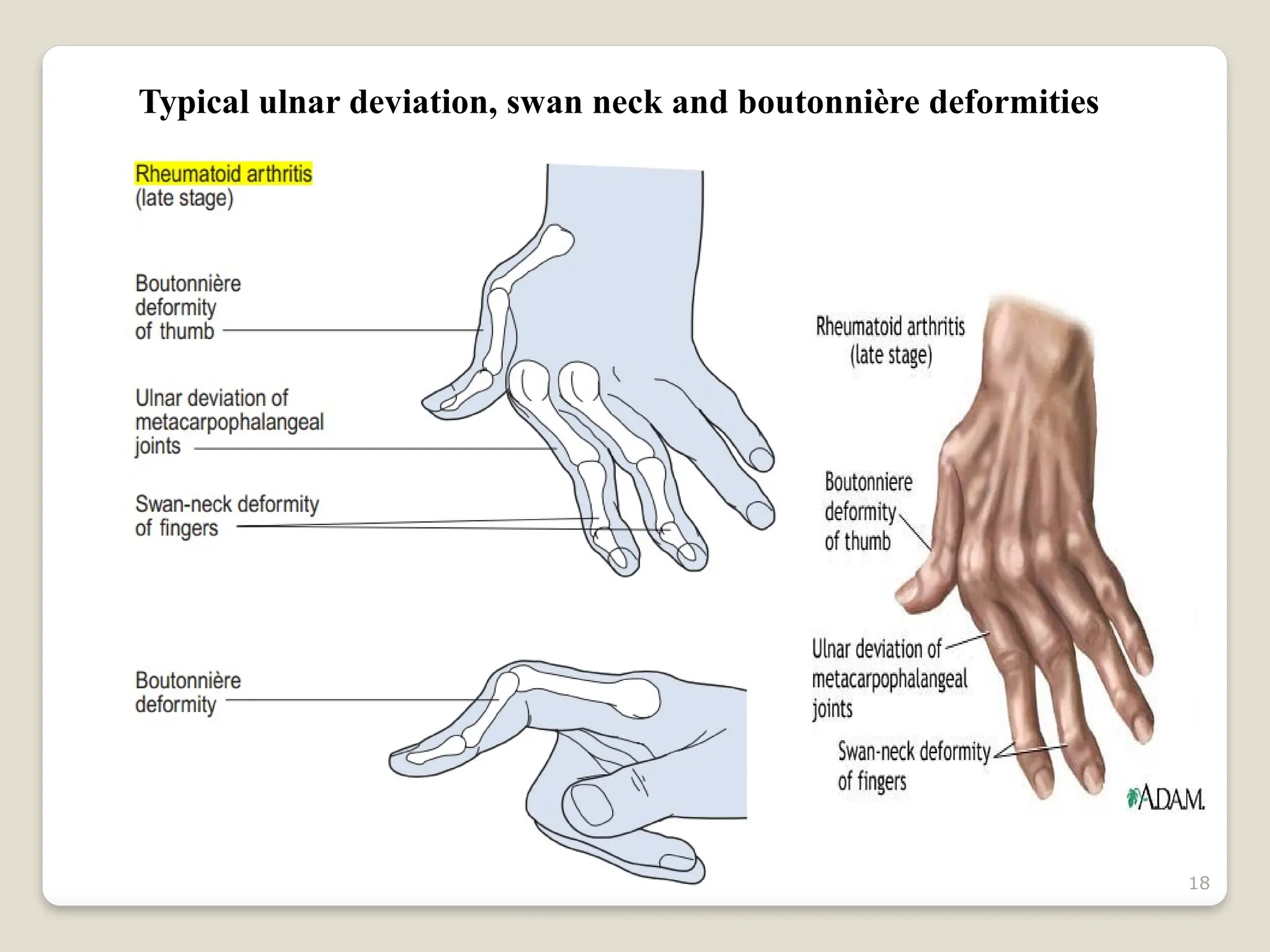
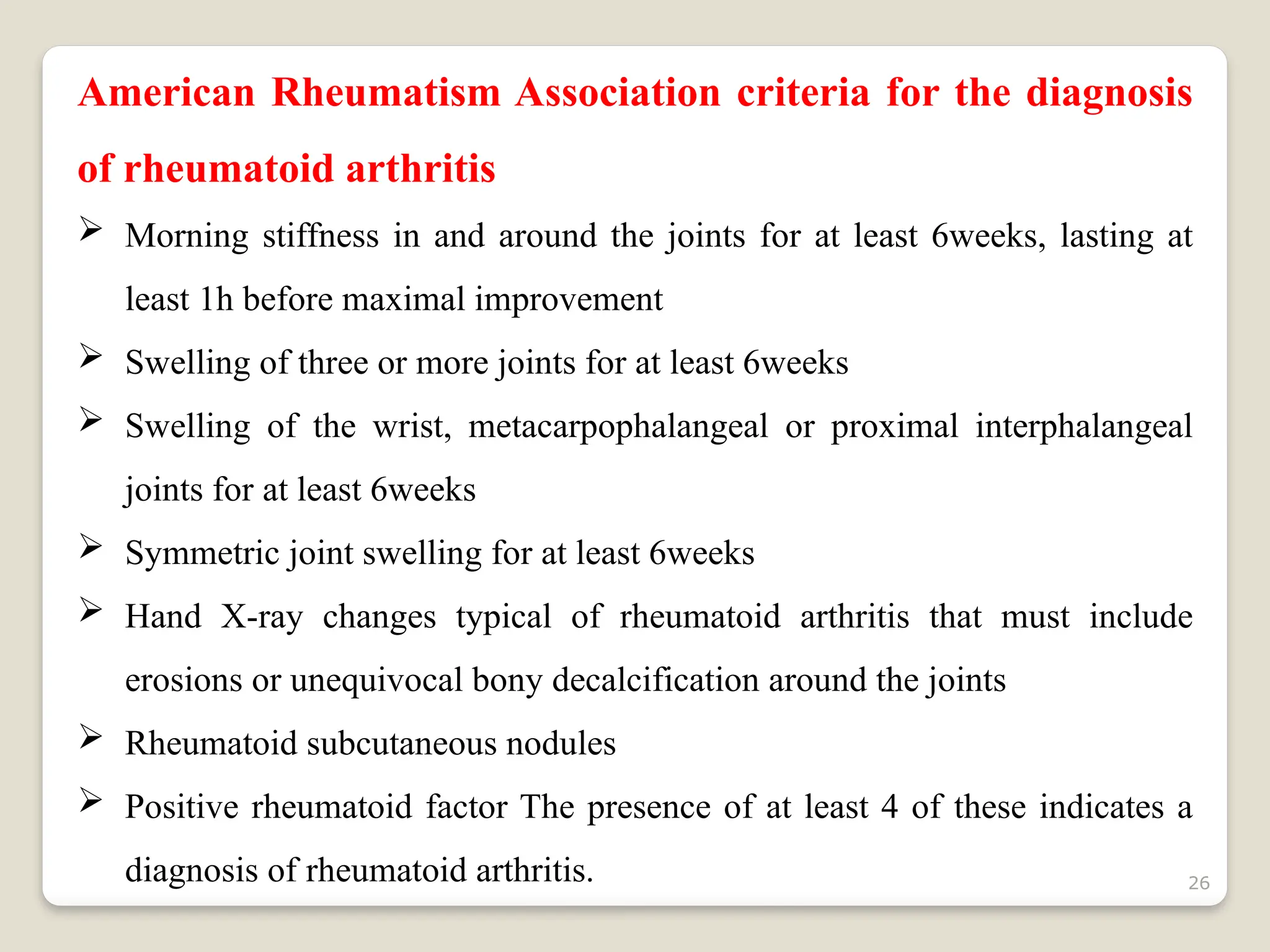
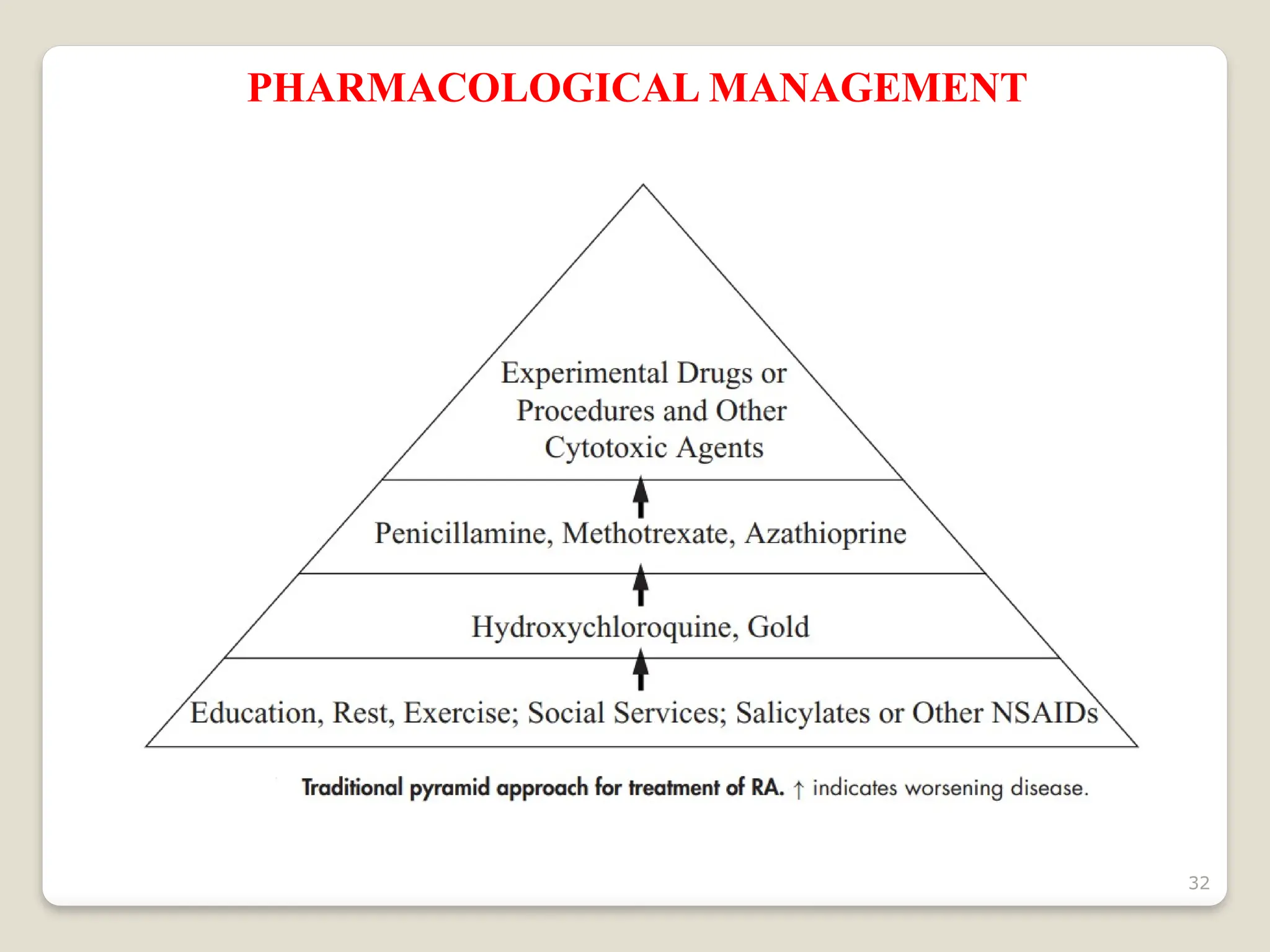
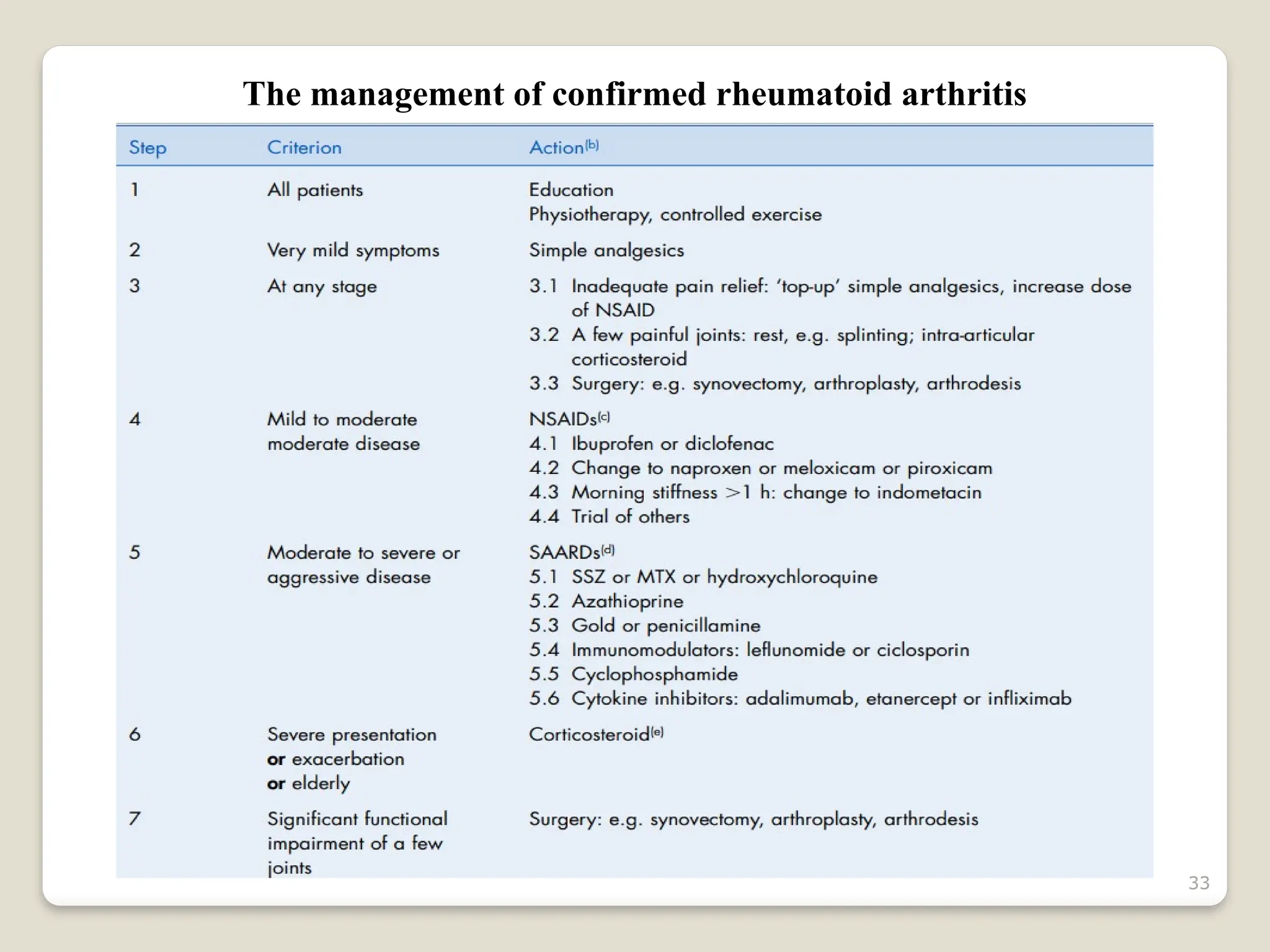
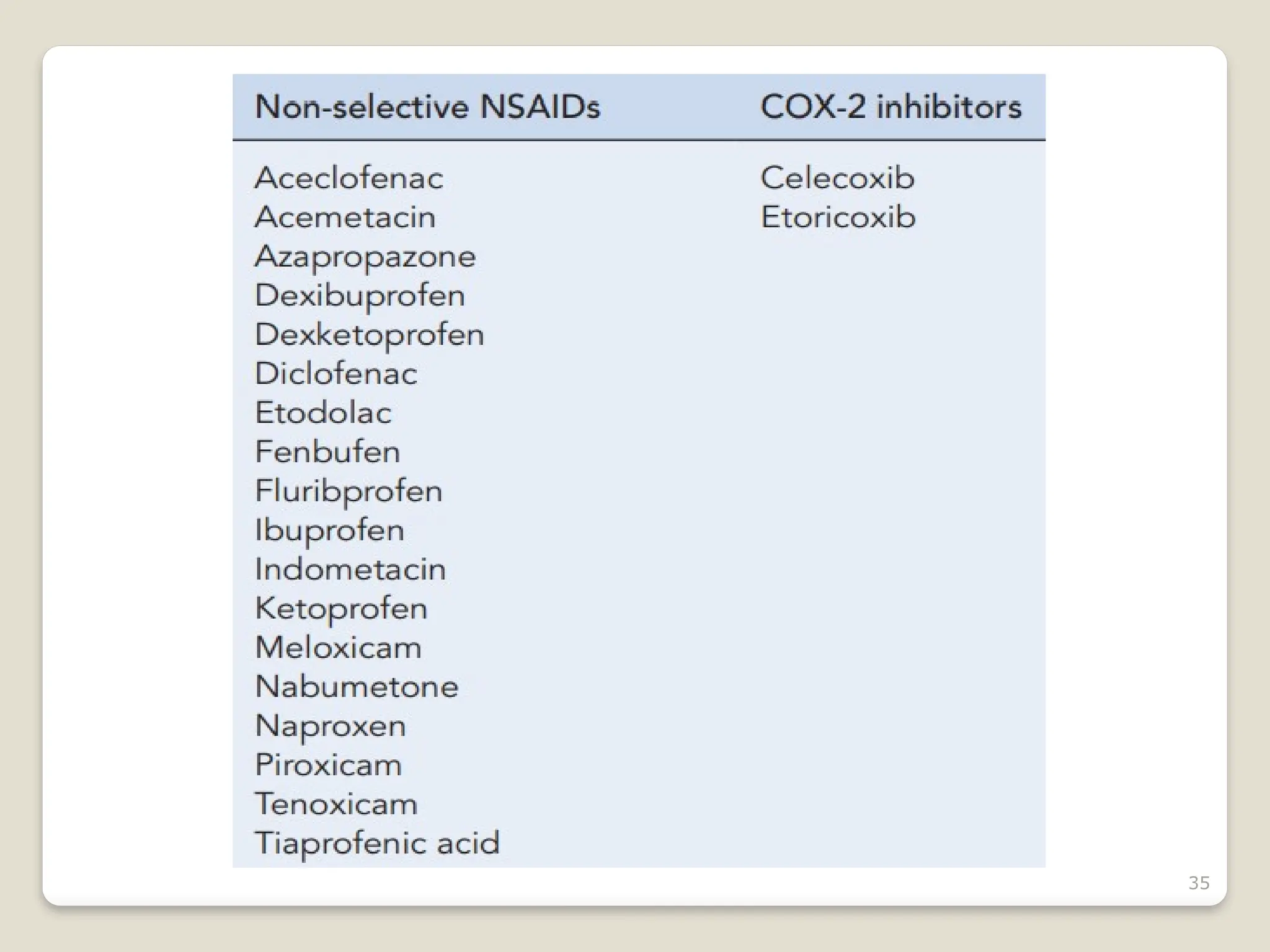
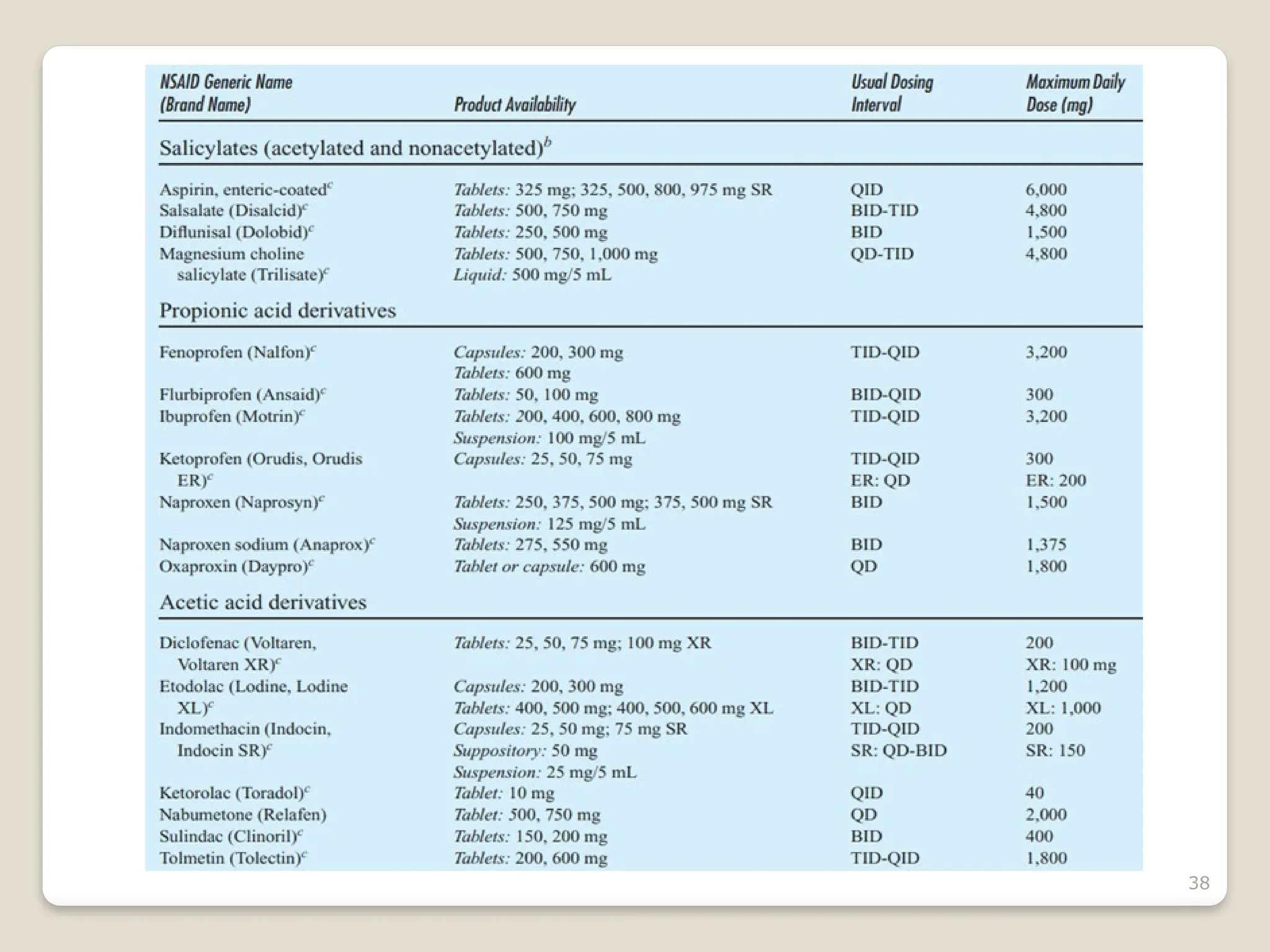
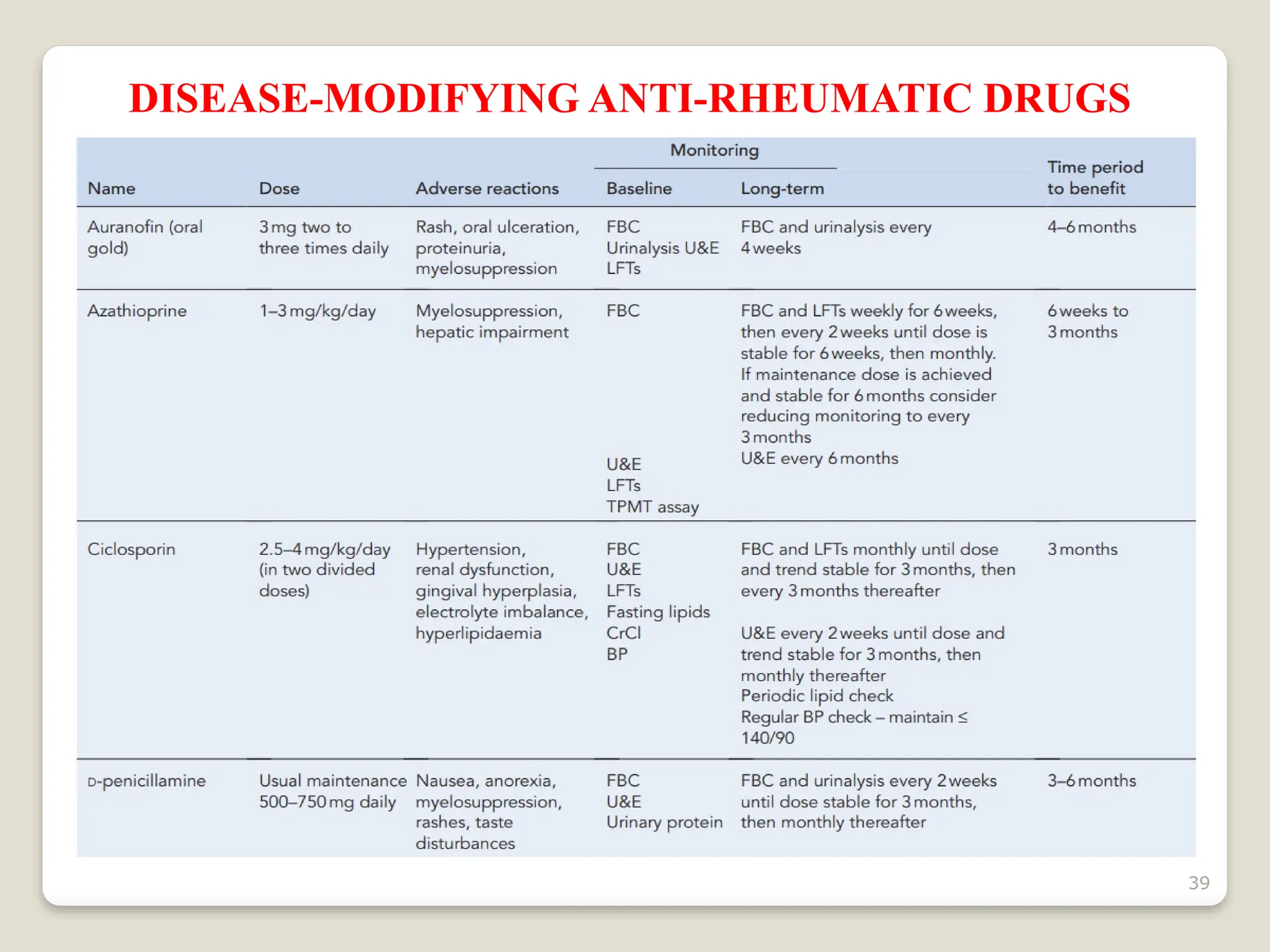
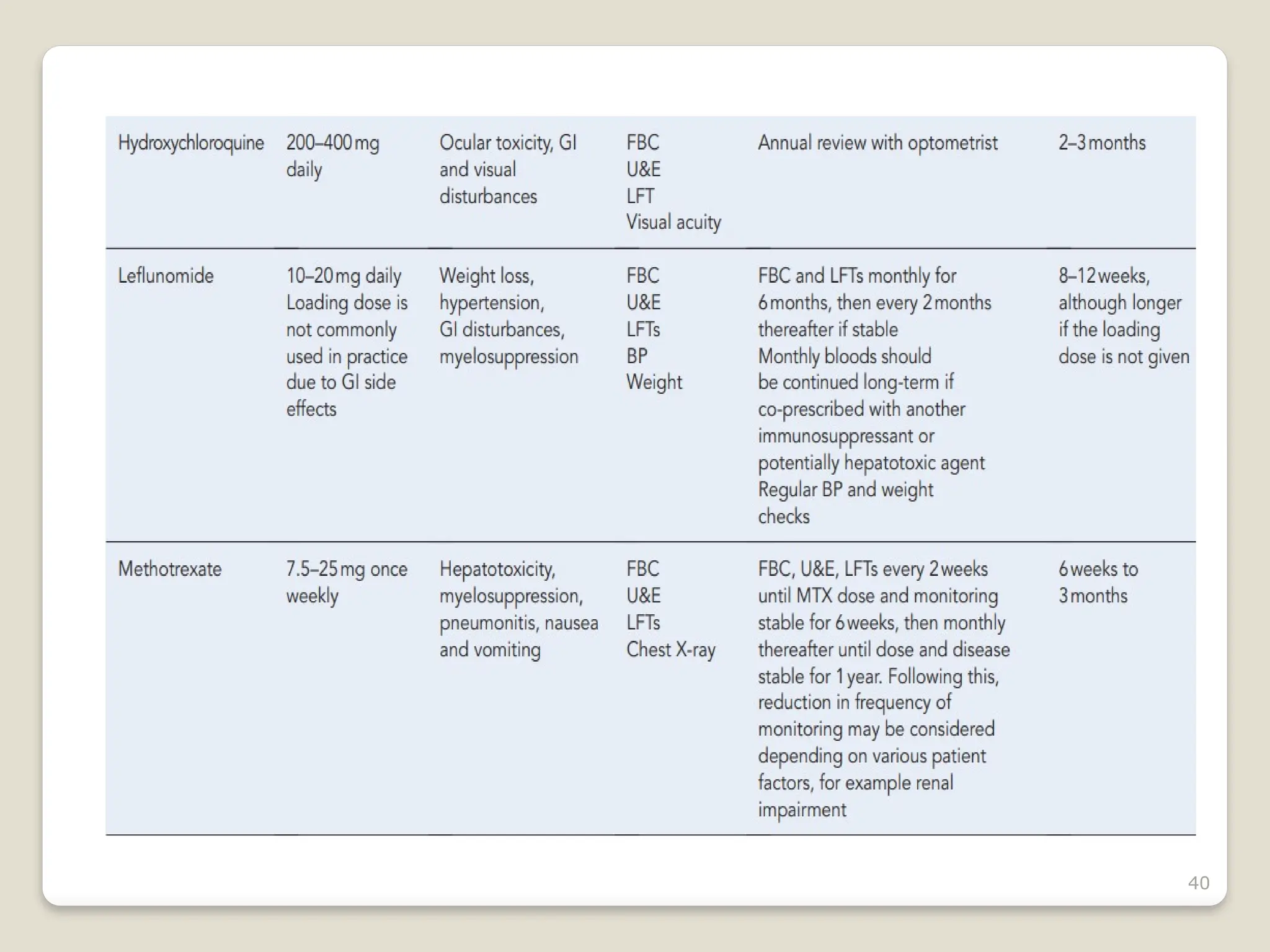
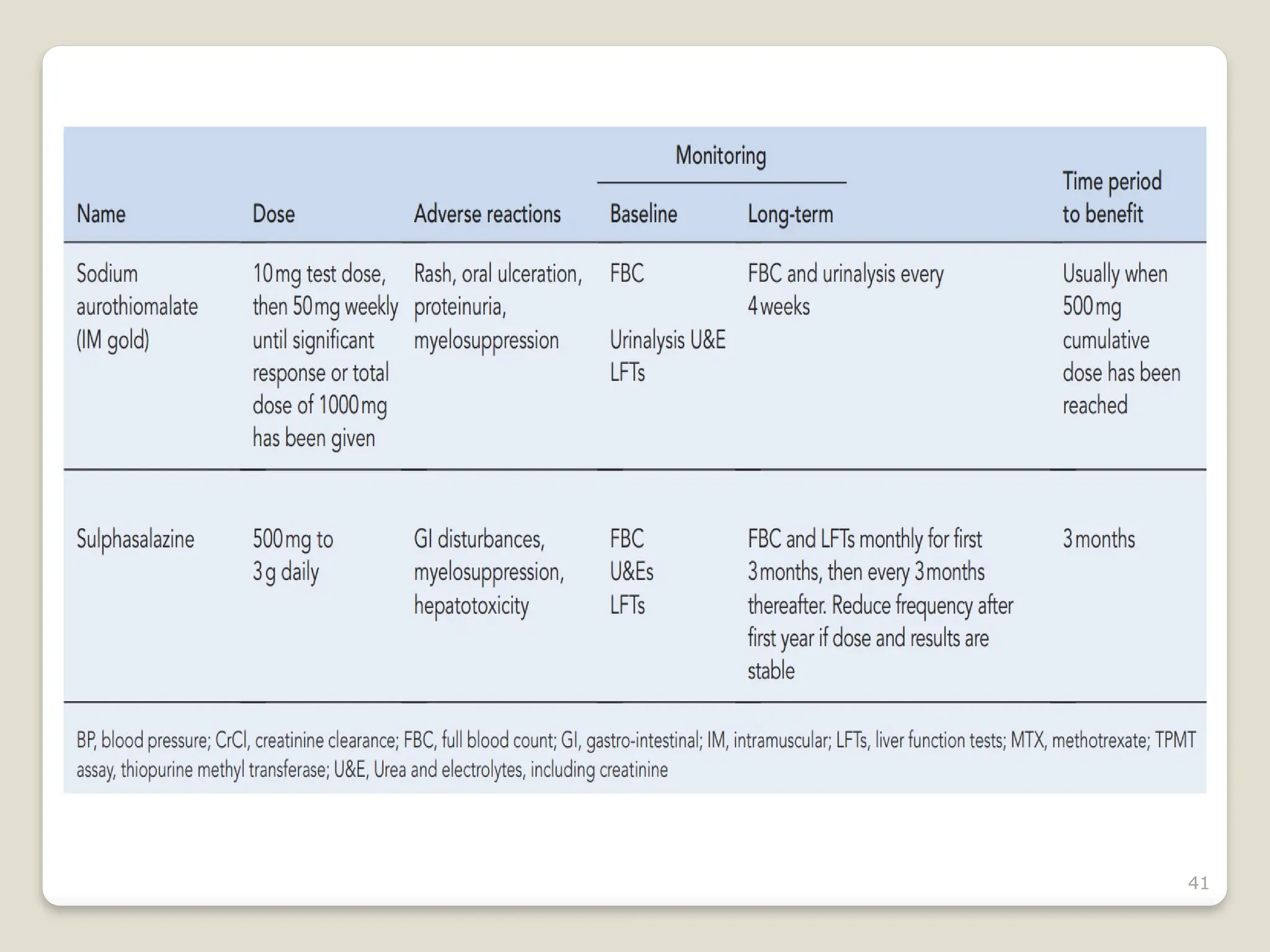
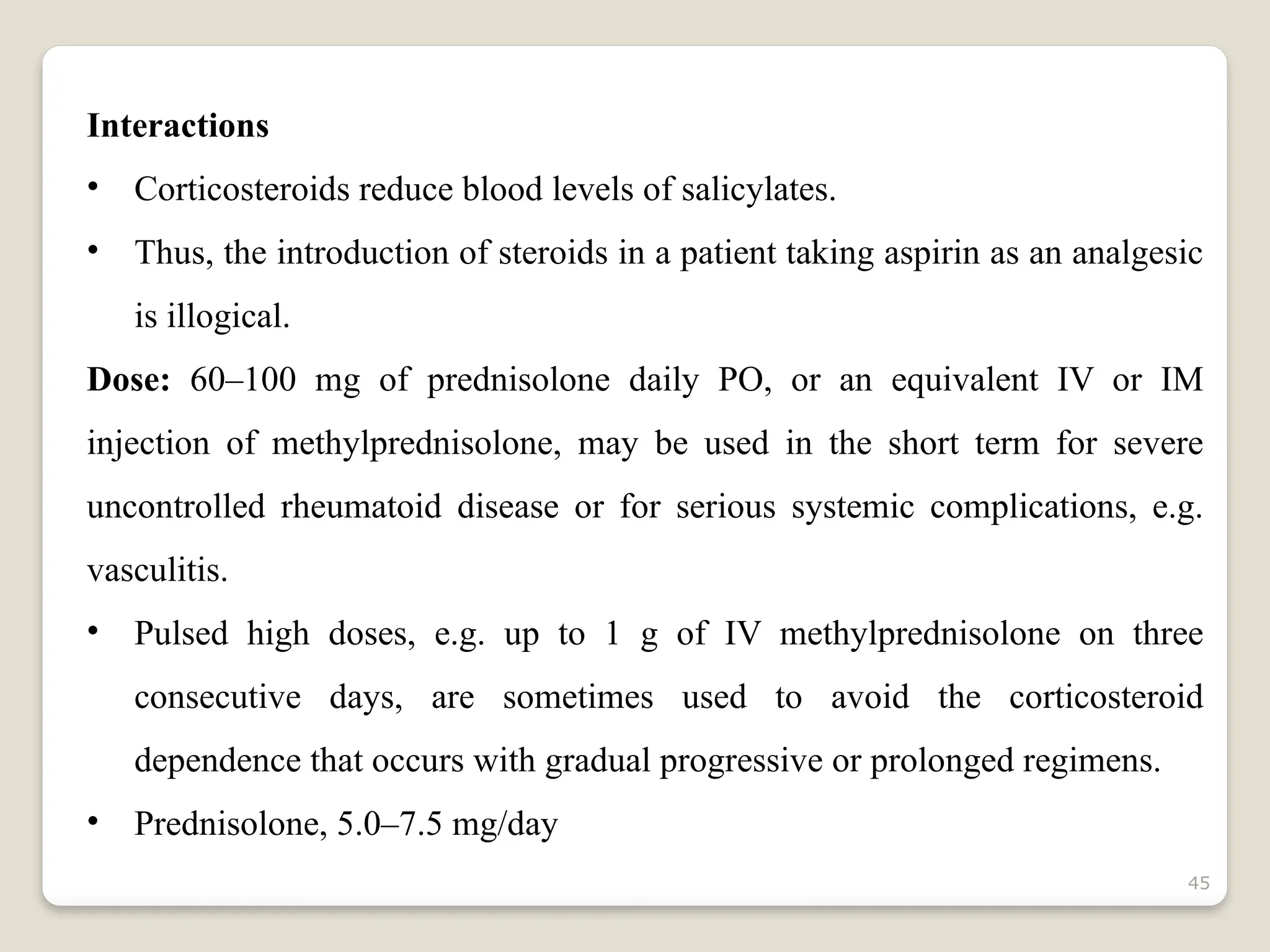
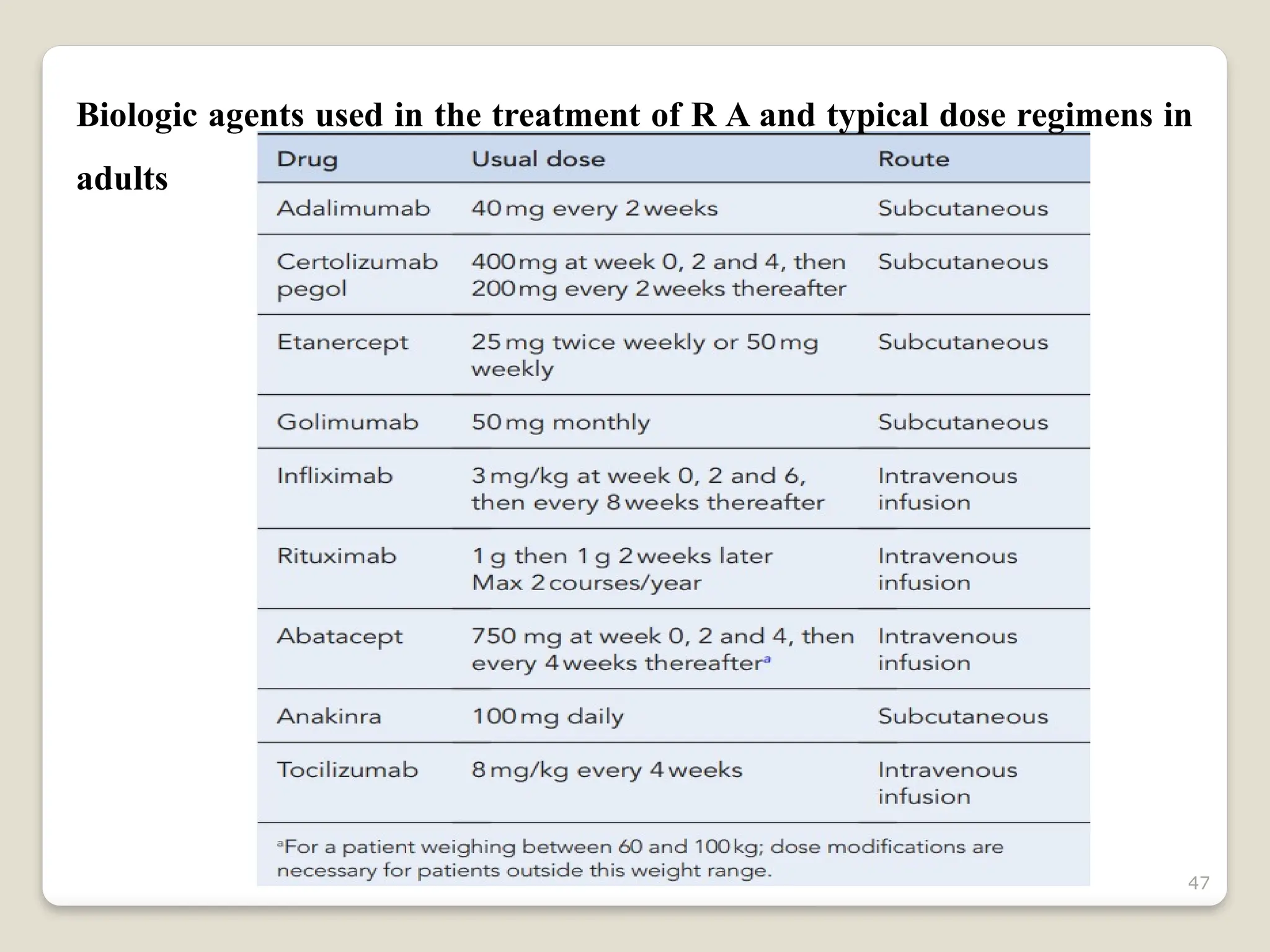
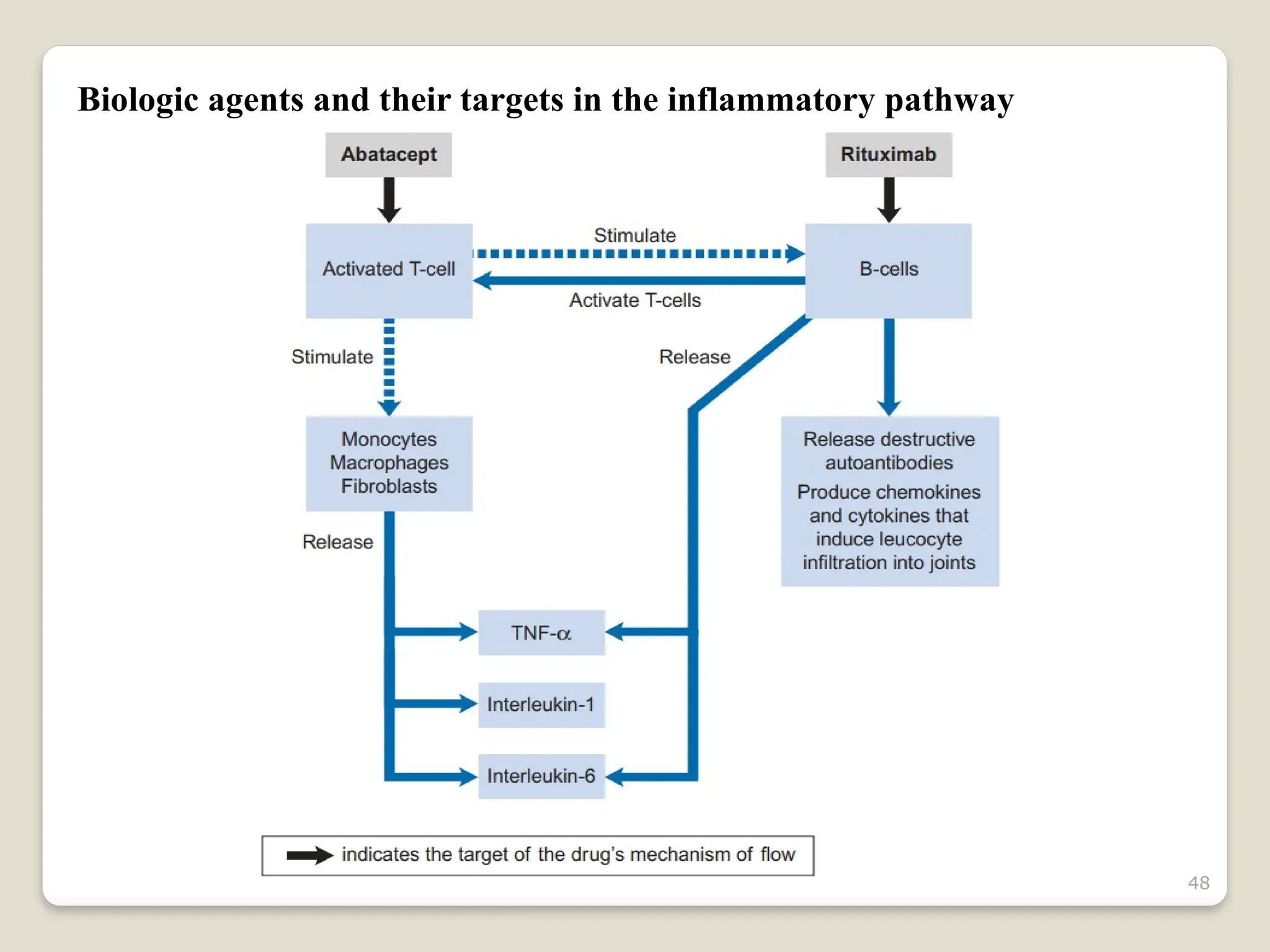
Rheumatoid arthritis (RA) is a chronic autoimmune disorder characterized by joint inflammation and systemic manifestations, affecting approximately 1% of the population, particularly women. The etiology of RA is unknown, but genetic, environmental, and lifestyle factors may contribute to its development, leading to severe joint deformity if untreated. Management focuses on symptom relief, preventing joint damage, and improving functional ability through a combination of non-pharmacological and pharmacological treatments, including NSAIDs, DMARDs, corticosteroids, and biologics.