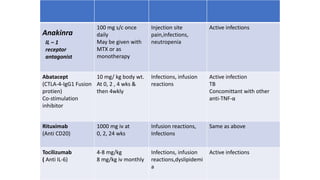
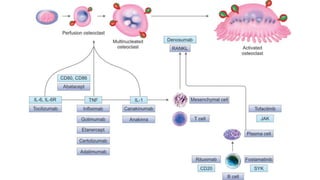
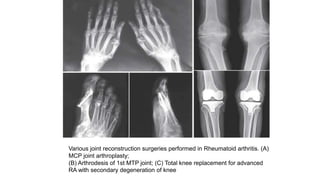
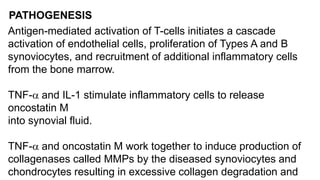
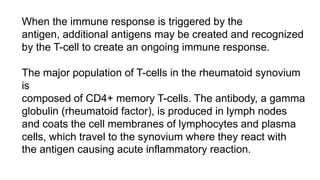
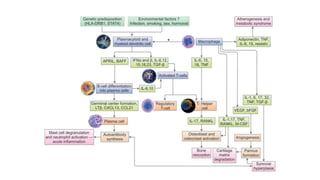
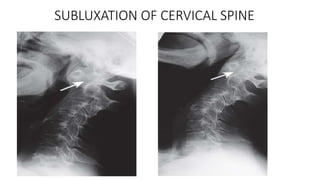
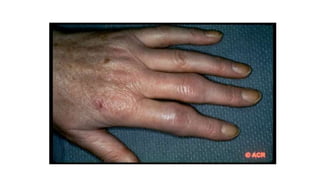
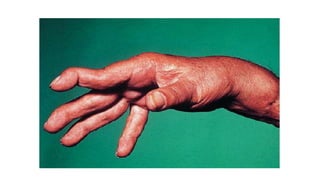
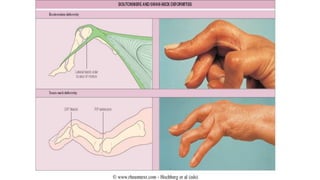
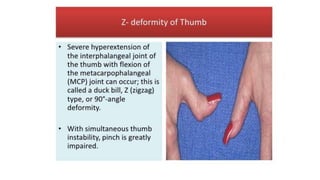
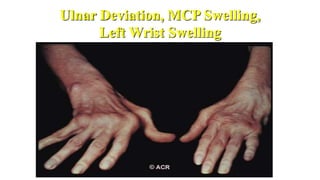
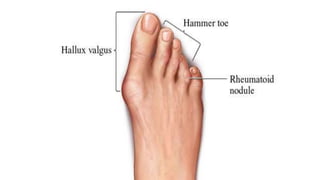
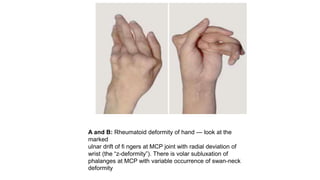
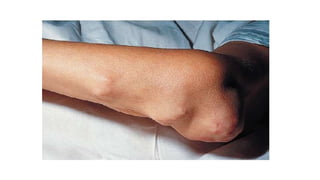
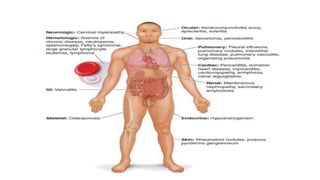
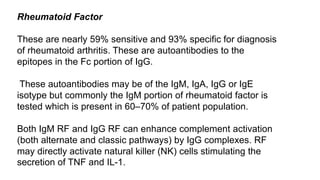
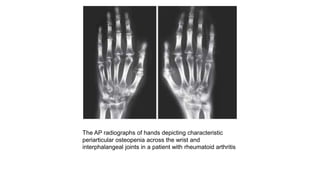
Rheumatoid arthritis (RA) is a chronic autoimmune disease characterized by symmetrical inflammatory polyarthritis, primarily affecting the small joints and more common in women. Diagnosis involves clinical symptoms, laboratory findings such as anti-CCP antibodies, and imaging studies to detect joint damage. Management focuses on pain relief, disease modification through DMARDs, and may include biologic therapies, with a need for patient education and individualized treatment plans.




















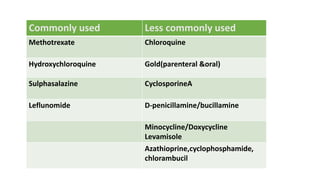
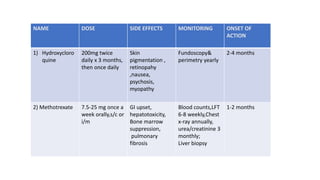
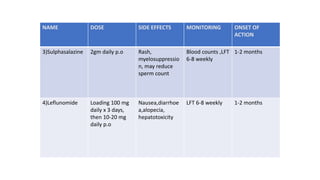
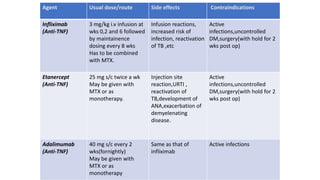
![[Previously Called Second Line Agents (They Are Now the
First Line Agents), Slow-acting Antirheumatic Drugs]—Now
Th ey Are Called “Synthetic” DMARDs (in View of
Availability
of Biological Agents)](https://image.slidesharecdn.com/rheumatoidarthritis-240611044303-c0c446f1/85/RHEUMATOID-ARTHRITIS-with-dmards-classification-pptx-39-320.jpg)