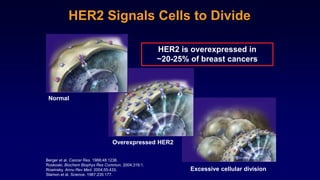
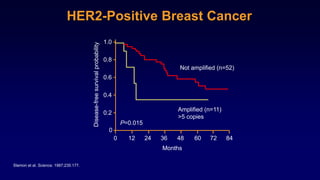
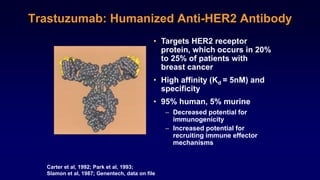
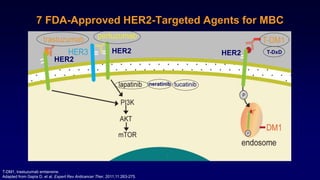
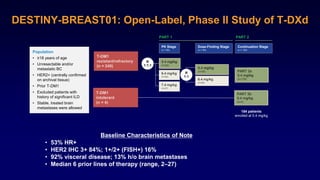
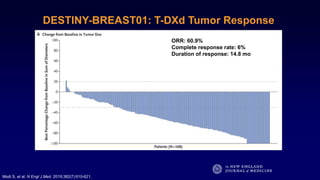
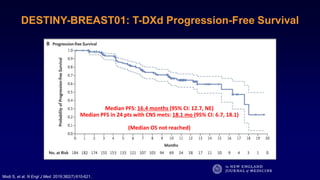
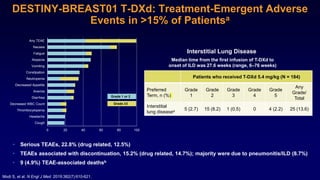
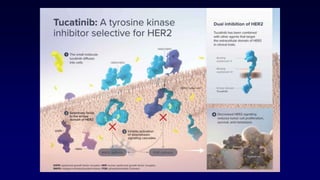
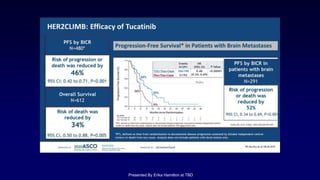
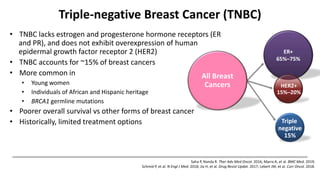
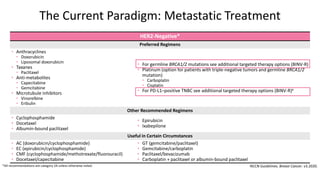
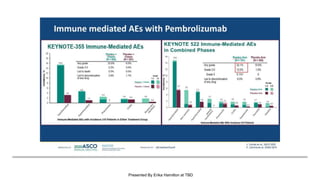
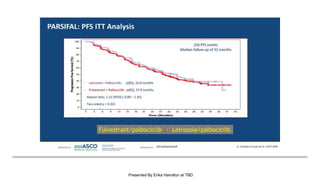
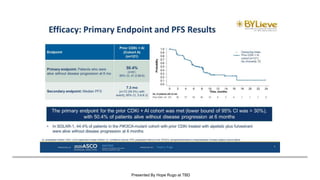
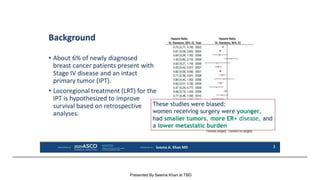
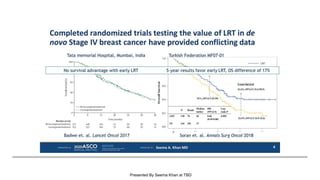
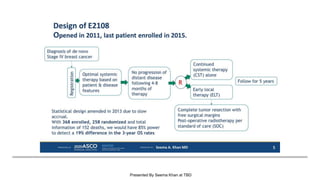
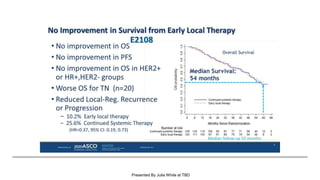
The document discusses updates in metastatic breast cancer treatment, highlighting HER2-positive, triple-negative, and ER-positive subtypes. It emphasizes new therapies such as fam-trastuzumab deruxtecan and tucatinib, their approval processes, and key clinical trial findings. It also touches on the importance of patient-doctor communication and engagement in treatment decisions.