The document outlines the key concepts of renal and gastrointestinal physiology, focusing on kidney structure and function, nephron components, and renal circulation. It details the mechanisms of filtration, reabsorption, and secretion in the kidneys, as well as the regulation of glomerular filtration rate (GFR) and renal blood flow. Important physiological processes such as micturition and the transport mechanisms at the nephron are also described.



























![Somatic
The amount of indicator filtered over time is
calculated as the plasma concentration of
the indicator (PIn, in g/L or mol/L) times the
GFR in L/min.
The same amount of indicator/time appears
in the urine and is calculated as V.U (in
L/min), times the indicator conc. in urine
(UIn, in g/L or mol/L, resp.), i.e.
PIn ⋅ GFR =V.U ⋅ UIn
or
GFR = (V . U ⋅ Uin)/Pin [L/min].
Glomerular Filtration and Clearance](https://image.slidesharecdn.com/tau-phs208renalgitlecture-2024-240618153601-669558b2/75/Renal-and-gastrointestinal-Physiology-pptx-28-2048.jpg)
















![Somatic
Na+/K+ transport by Na+-K+-ATPase in the
basolateral membrane of the tubule and collecting
duct serves as the “motor” for most of these
transport processes.
By primary active transport (fuelled directly by ATP
consumption), Na+ - K+ - ATPase pumps Na+ out of
the cell into the blood while pumping K+ in the
opposite direction (subscript “i” = intracellular and “o”
= extracellular).
This creates two driving “forces” essential for the
transport of numerous substances (including Na+
and K+):
first, a chemical Na+ gradient ([Na+]o [Na+]i and
(because [K+]i >[K+]o)
second, a membrane potential (inside the cell is
negative relative to the outside) which represents an
electrical gradient and can drive ion transport.
Transport Processes at the Nephron](https://image.slidesharecdn.com/tau-phs208renalgitlecture-2024-240618153601-669558b2/75/Renal-and-gastrointestinal-Physiology-pptx-45-2048.jpg)
























![Somatic
Reabsorption of Water, Formation of Concentrated Urine
The glomeruli filter around 180 L of plasma water each day (= GFR).
By comparison, the normal urine output (V.U) is relatively small (0.5 to 2
L/day).
Normal fluctuations are called antidiuresis (low V.U) and diuresis (high
V.U).
Urine output above the range of normal is called polyuria.
Below normal output is defined as oliguria (0.5 L/day) or anuria (0.1 L/day).
The osmolality of plasma and glomerular filtrate is about 290
mOsm/kgH2O (=Posm); that of the final urine (Uosm) ranges from50
(hypotonic urine in extreme water diuresis) to about 1200mOsm/kg H2O
(hypertonic urine in maximally concentrated urine).
Since water diuresis permits the excretion of large volumes of H2O without
the simultaneous loss of NaCl and other solutes, this is known as
“freewater excretion”, or “free water clearance” (CH2O).
This allows the kidneys to normalize decreases in plasma osmolality, for
example.
The CH2O represents the volume of water that could be theoretically
extracted in order for the urine to reach the same osmolality as the plasma:
CH2O V.U (1–[Uosm/Posm]).](https://image.slidesharecdn.com/tau-phs208renalgitlecture-2024-240618153601-669558b2/75/Renal-and-gastrointestinal-Physiology-pptx-70-2048.jpg)













![Body Fluid Homeostasis
Water is the initial and final product of countless biochemical reactions.
It serves as a solvent, transport vehicle, heat buffer, coolant, and has a
variety of other functions.
Water is present in cells as an intracellular fluid.
The volume of fluid circulating in the body remains relatively constant
when the water balance is properly regulated. The average fluid
intake of ca. 2.5 L per day is supplied by beverages, solid foods, and
metabolic oxidation.
The fluid intake must be high enough to counteract water losses due
to urination, respiration, perspiration, and defecation.
The mean daily H2O turnover is 2.5 L/70 kg (1/30 th the bodyweight
[BW]) in adults and 0.7 liters/7 kg (1/10th the BW) in infants.
The water balance of infants is, therefore, more susceptible to
disturbance.
Significant rises in the H2O turnover can occur, but must be
adequately compensated for if the body is to function properly.
Respiratory H2O losses occur, for example, due to hyperventilation at
high altitudes, and perspiration losses occur due to exertion at high
temperatures (e.g., hiking in the sun or hot work environment as in an
ironworks).
Somatic](https://image.slidesharecdn.com/tau-phs208renalgitlecture-2024-240618153601-669558b2/75/Renal-and-gastrointestinal-Physiology-pptx-84-2048.jpg)


![Somatic
Provided the indicator substance, S injected into the bloodstream spreads to
the target compartment only (C), its volume V can be calculated from:
V[L] = injected amount of indicator S [mol]/CS [mol/L] [7.12] where CS is
the concentration of S after it spreads throughout the target compartment
(measured in collected blood specimens).
The ECF volume is generally measured using inulin or sodium bromide as the
indicator (does not enter cells), and the TBW volume is determined using
antipyrine, heavy water (D2O), or radiolabelled H2O.
The ICF volume is approximately equal to the antipyrine distribution volume
minus the inulin distribution volume.
Radiolabelled albumin or Evans blue, a substance entirely bound by plasma
proteins, can be used to measure the plasma volume.
The interstitial volume can be calculated as the ECF volume minus the plasma
volume, and the blood volume as the plasma volume divided by [1–hematocrit].
(Since 1/10 of the plasma remains interspersed with the erythrocytes after
centrifuging, replacing 1 with 0.91 in this formula gives a more precise result.)
The blood volume can also be determined by injection of 51Cr-marked
erythrocytes so that the plasma volume is then calculated as blood volume
times (0.91– Hct).
Body Fluid Homeostasis](https://image.slidesharecdn.com/tau-phs208renalgitlecture-2024-240618153601-669558b2/75/Renal-and-gastrointestinal-Physiology-pptx-87-2048.jpg)









































![Somatic
Tubuloglomerular Feedback, Renin–Angiotensin System
When less is absorbed upstream, the urine flows more quickly through the
thick ascending limb of the loop, resulting in a lower extent of urine dilution and
a higher NaCl concentration at the macula densa, [NaCl]MD.
If the [NaCl] MD becomes too high, the afferent arteriole will constrict to curb
the GFR of the affected nephron within 10 s or vice versa (negative feedback).
It is unclear how the [NaCl]MD results in the signal to constrict, but type 1 A
angiotensin II (AT1A) receptors play a key role.
If, however, the [NaCl]MD changes due to chronic shifts in total body NaCl
and an associated change in ECF volume, rigid coupling of the SNGFR with
the [NaCl]MD through TGF would be fatal.
Since long term increases in the ECF volume reduce proximal NaCl
reabsorption, the [NaCl]MD would increase, resulting in a decrease in the GFR
and a further increase in the ECF volume.
The reverse occurs in ECF volume deficit.
To prevent these effects, the [NaCl]MD/SNGFR response curve is shifted in
the appropriate direction by certain substances.
Nitric oxide (NO) shifts the curve when there is an ECF excess (increased
SNGFR at same [NaCl]MD), and (only locally effective) angiotensin II shifts it in
the other direction when there is an ECF deficit.](https://image.slidesharecdn.com/tau-phs208renalgitlecture-2024-240618153601-669558b2/75/Renal-and-gastrointestinal-Physiology-pptx-129-2048.jpg)













































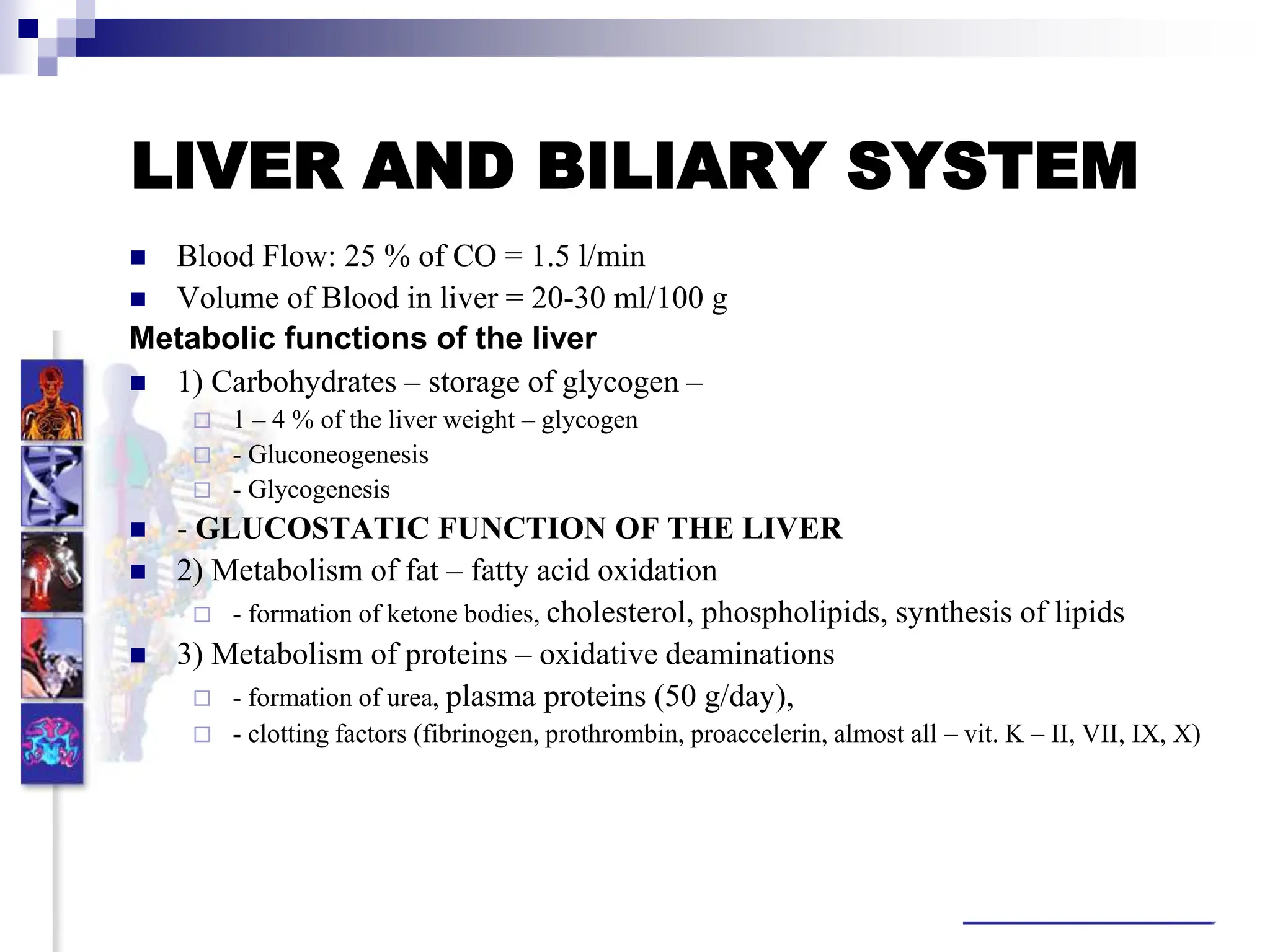































































































![ Pancreatic juice secretion is controlled in the acini
by cholinergic (vagal) mechanisms and by the
hormone cholecystokinin (CCK, vagal stimulation
seems to be enhanced by CCKA receptors).
Both cause an elevation of cytosolic concentration of
Ca2, [Ca2]i, which stimulates Cl– and (pro-)enzyme
secretion.
Trypsin in the small intestinal lumen deactivates
CCK release via a feedback loop.
Secretin increases HCO3– and H2O secretion by
the ducts.
CCK and vagal acetylcholine (ACh) potentiate this
effect by raising [Ca2]i.
The hormones also have a growth-promoting
effect.
Pancreas
Somatic](https://image.slidesharecdn.com/tau-phs208renalgitlecture-2024-240618153601-669558b2/75/Renal-and-gastrointestinal-Physiology-pptx-271-2048.jpg)




