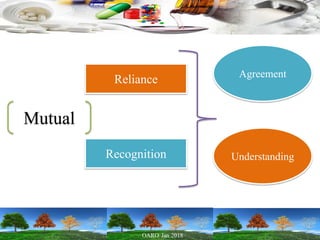
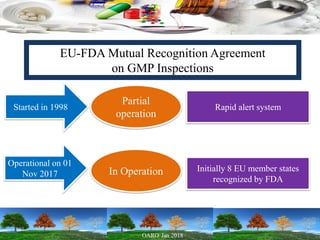
Cooperation between regulatory authorities is essential for managing the complex global drug supply chain. The EU and FDA signed a mutual recognition agreement on GMP inspections in 1998 to increase efficiency and oversight of drug manufacturing inspections. Starting in November 2017, the agreement fully took effect, allowing the EU and FDA to rely on each other's GMP inspections for drug manufacturers in initially 8 EU member states, reducing duplication of inspections. The agreement is aimed at strengthening inspections and oversight while allowing for a more practical management of resources.