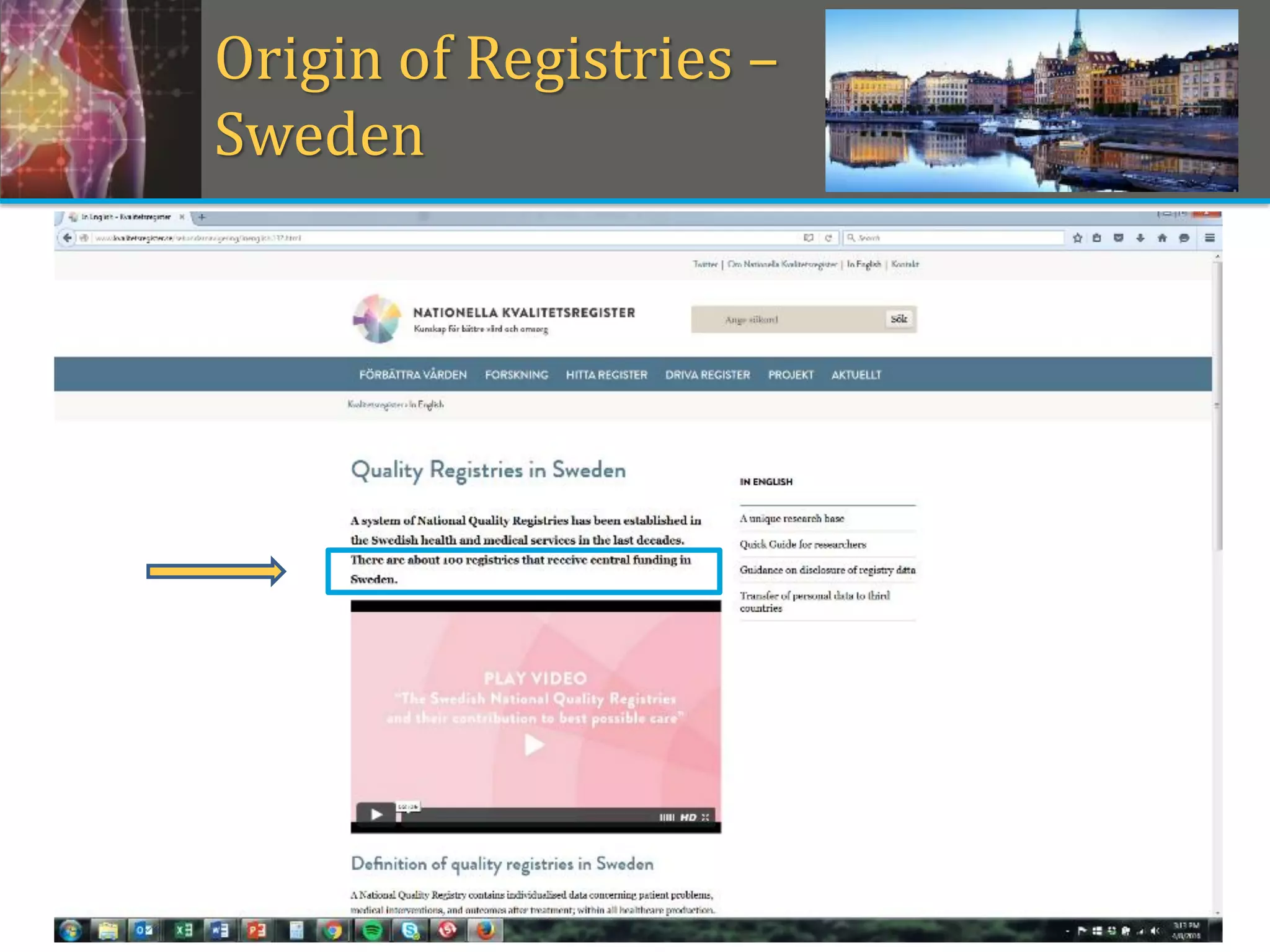
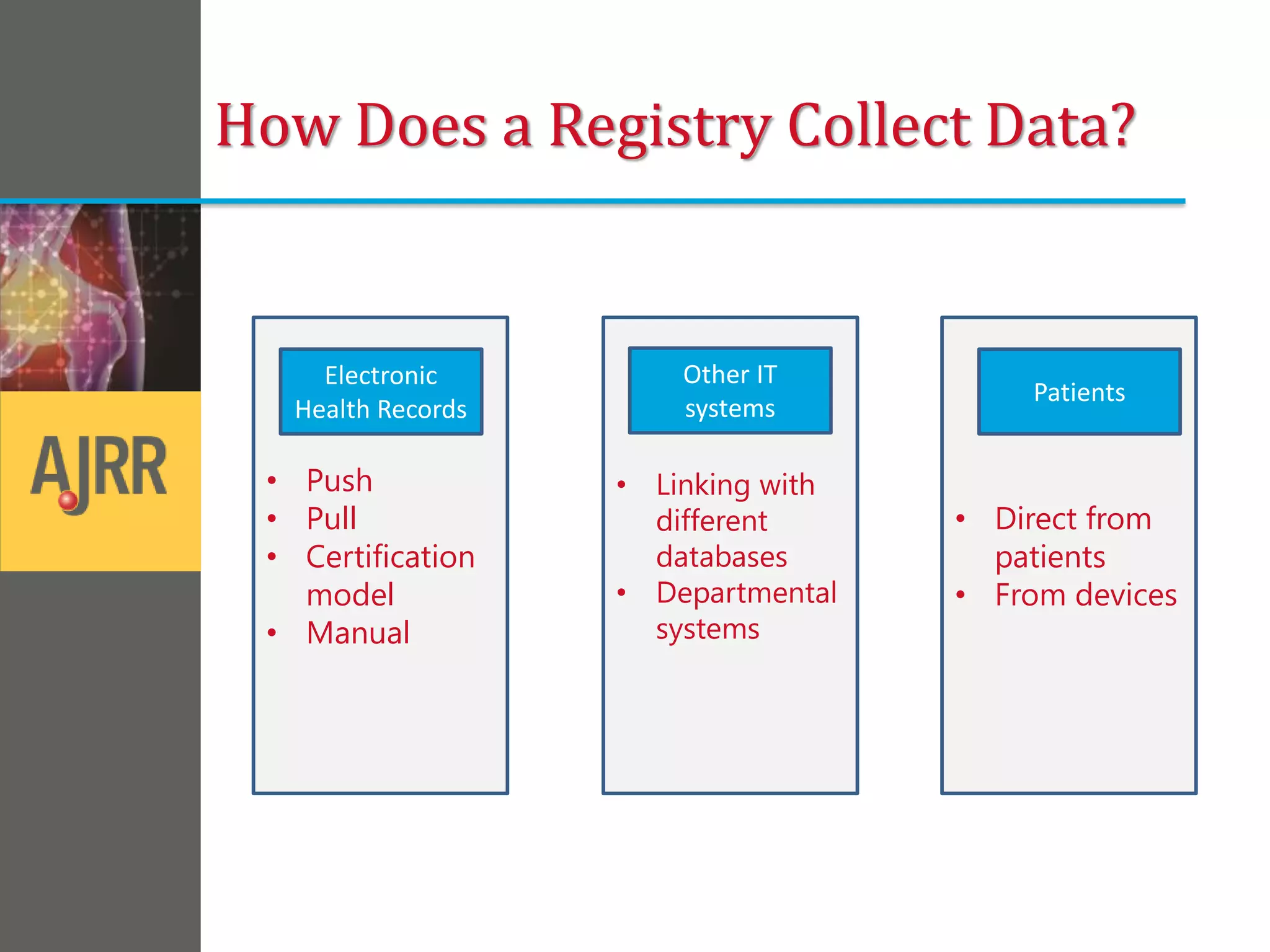
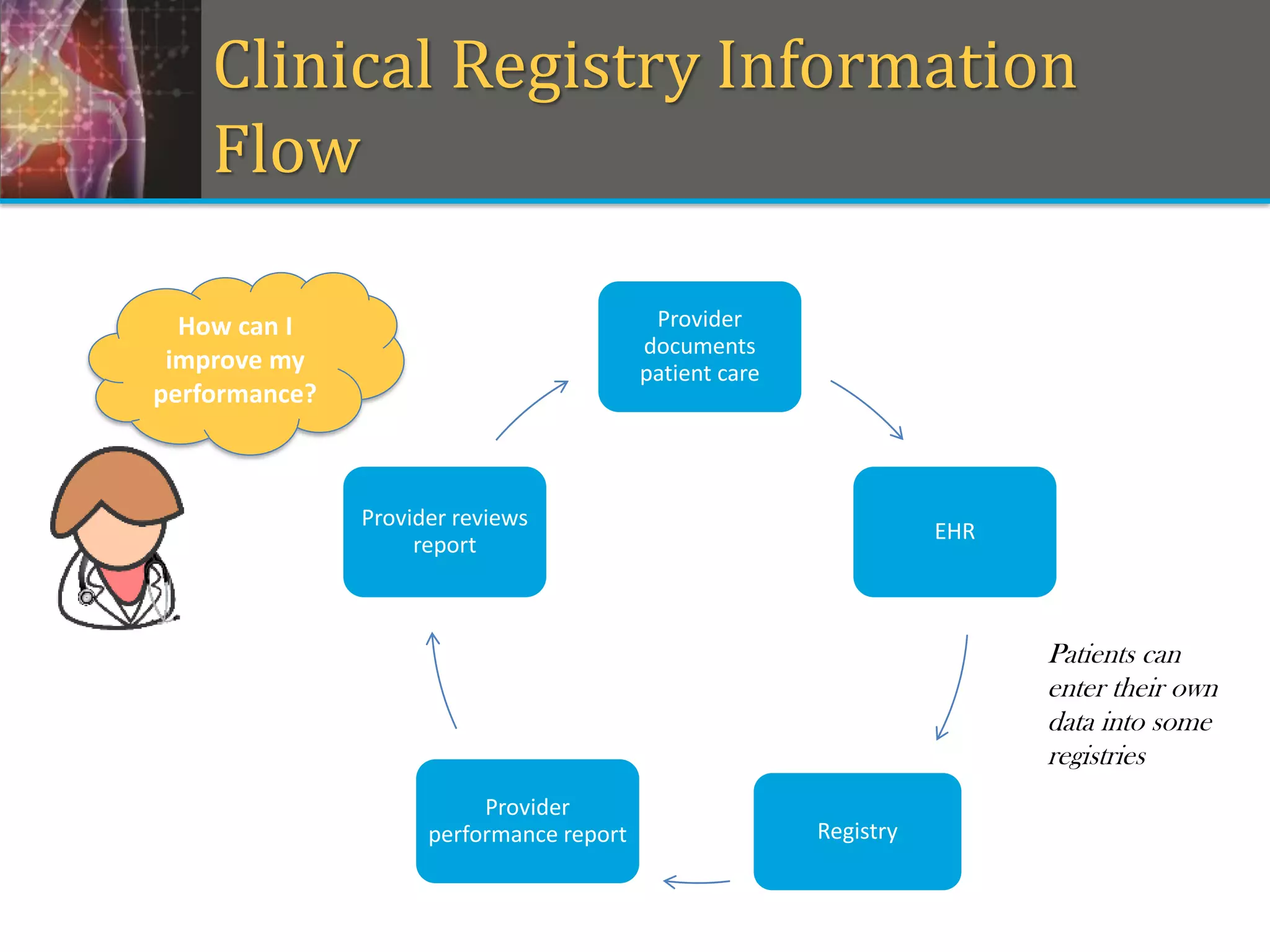
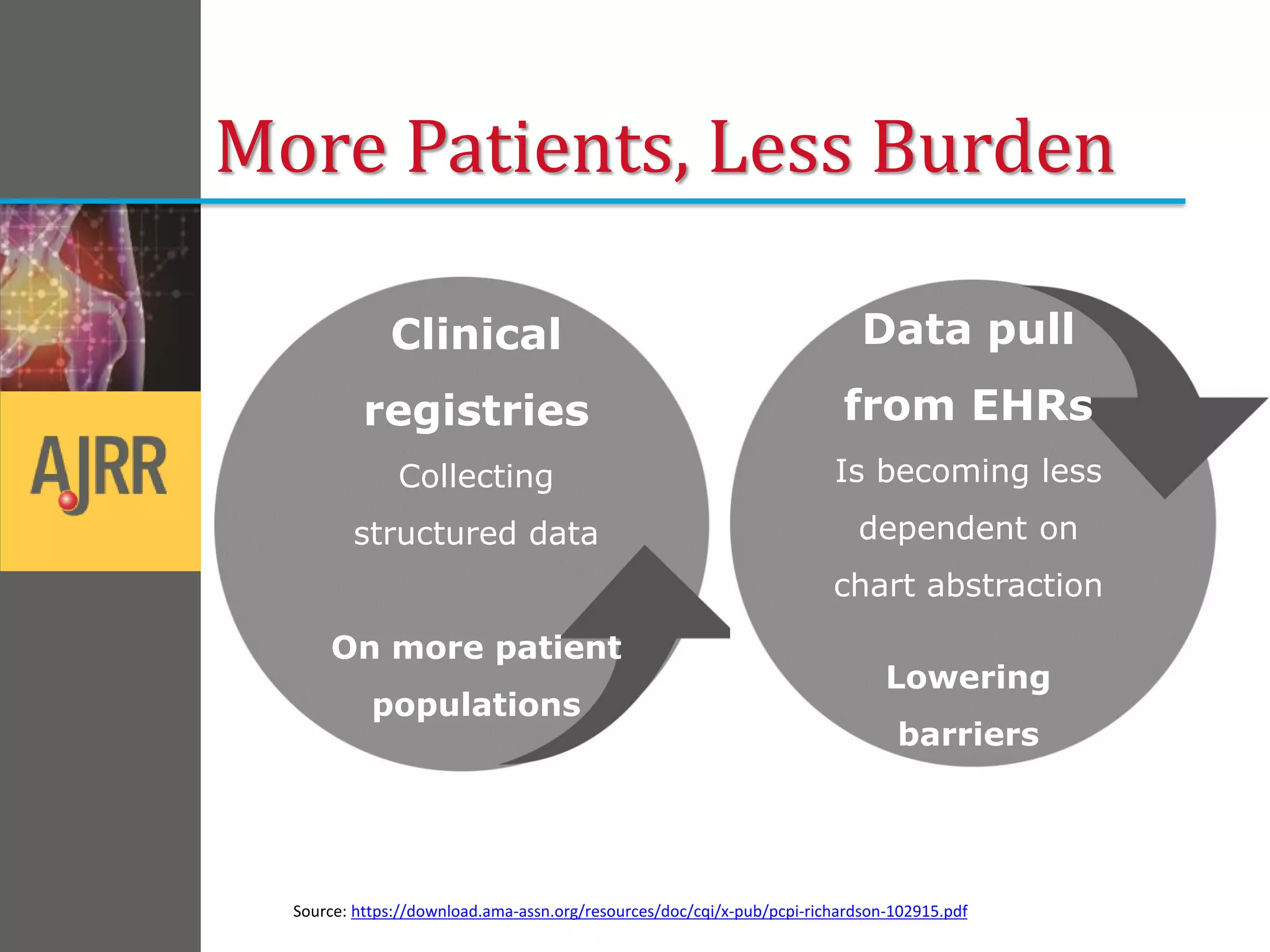
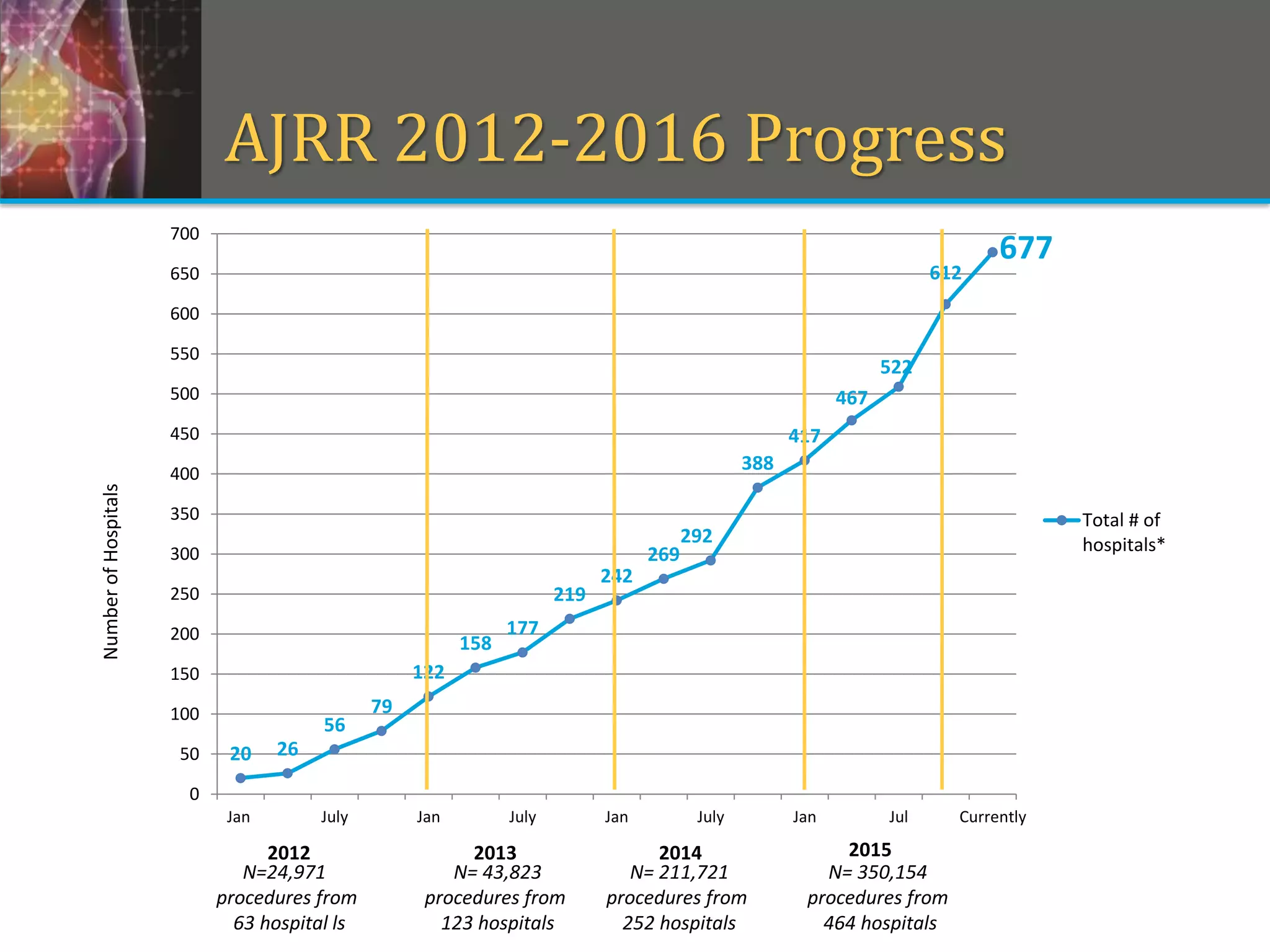
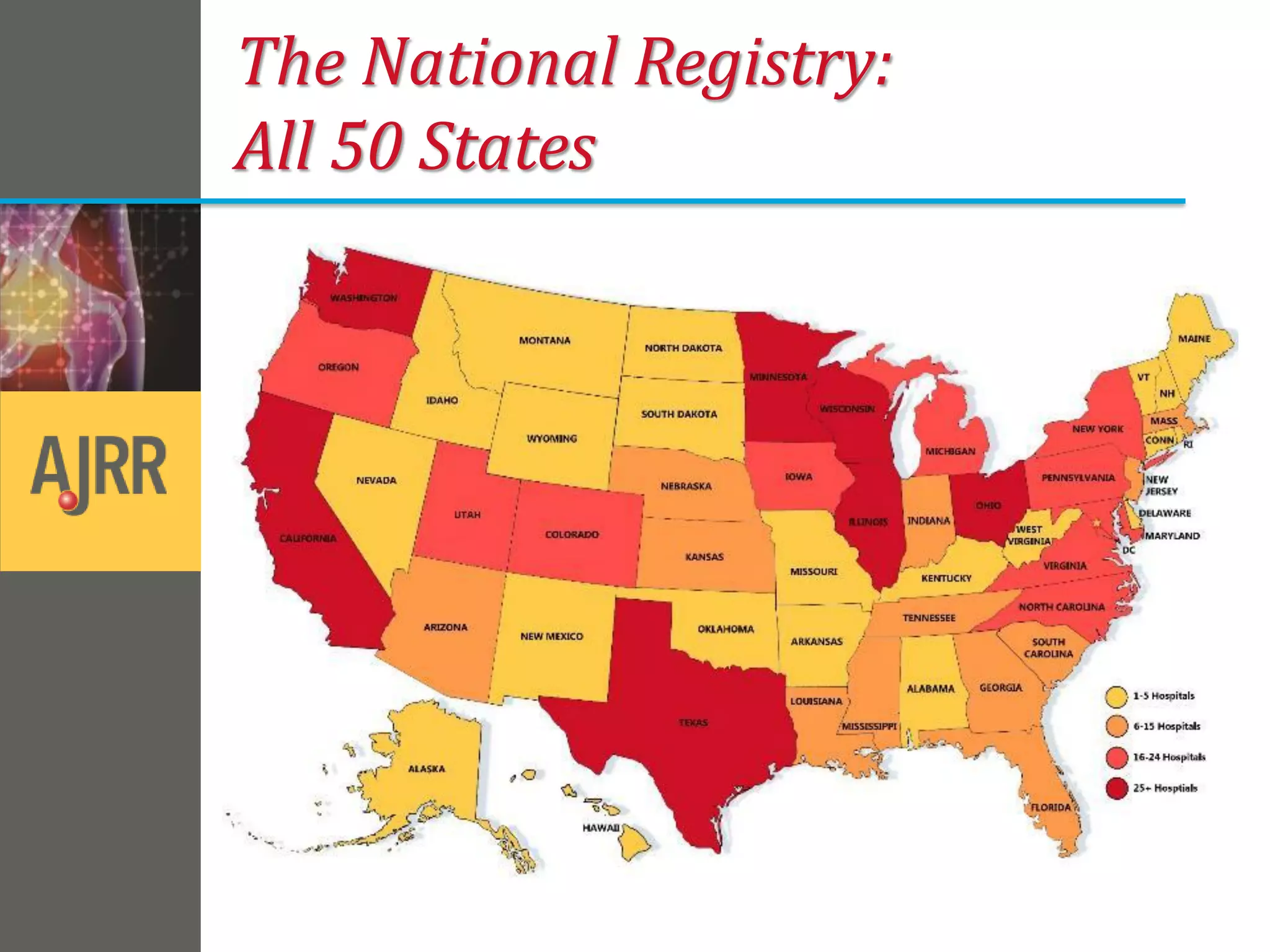
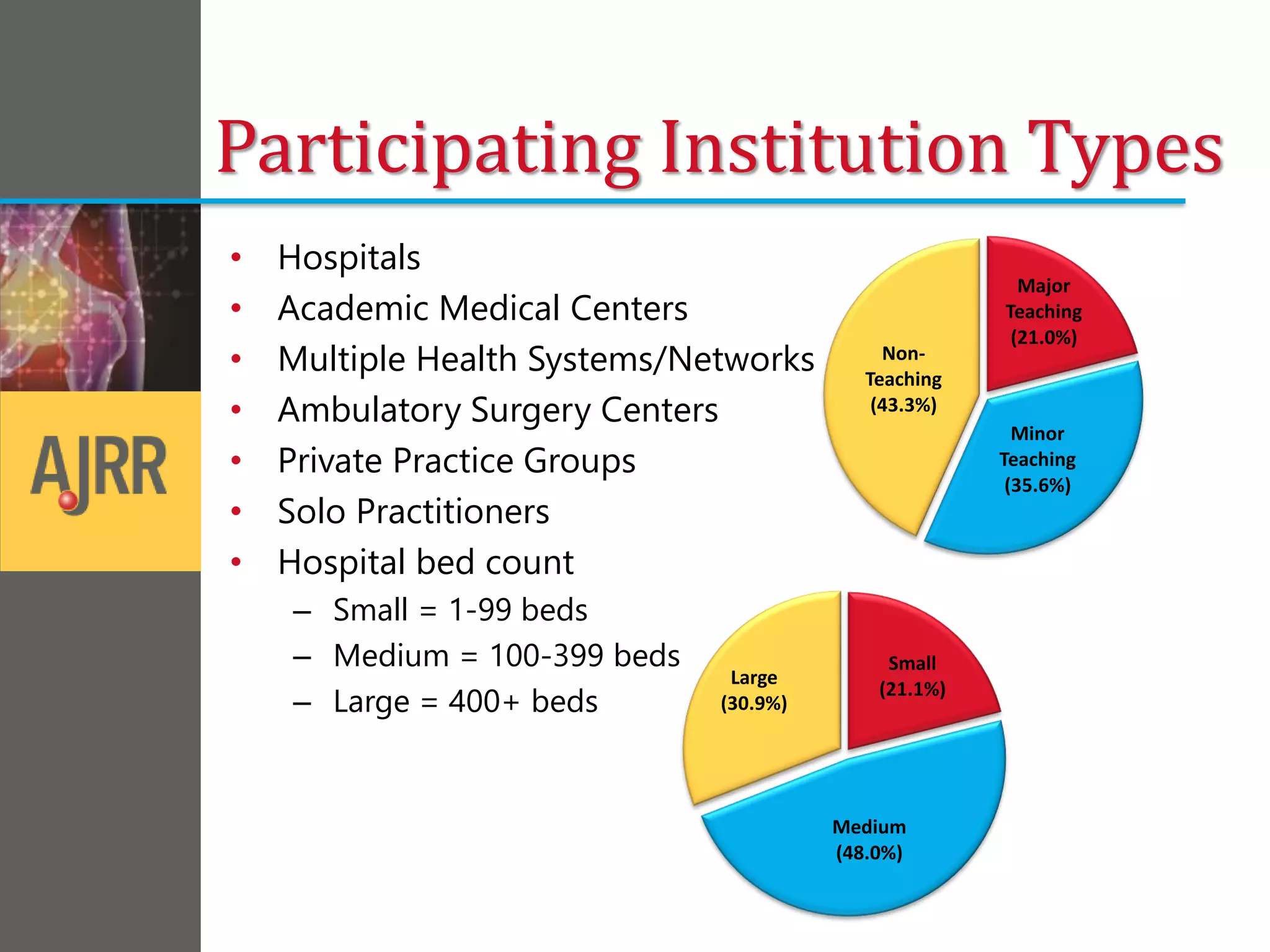
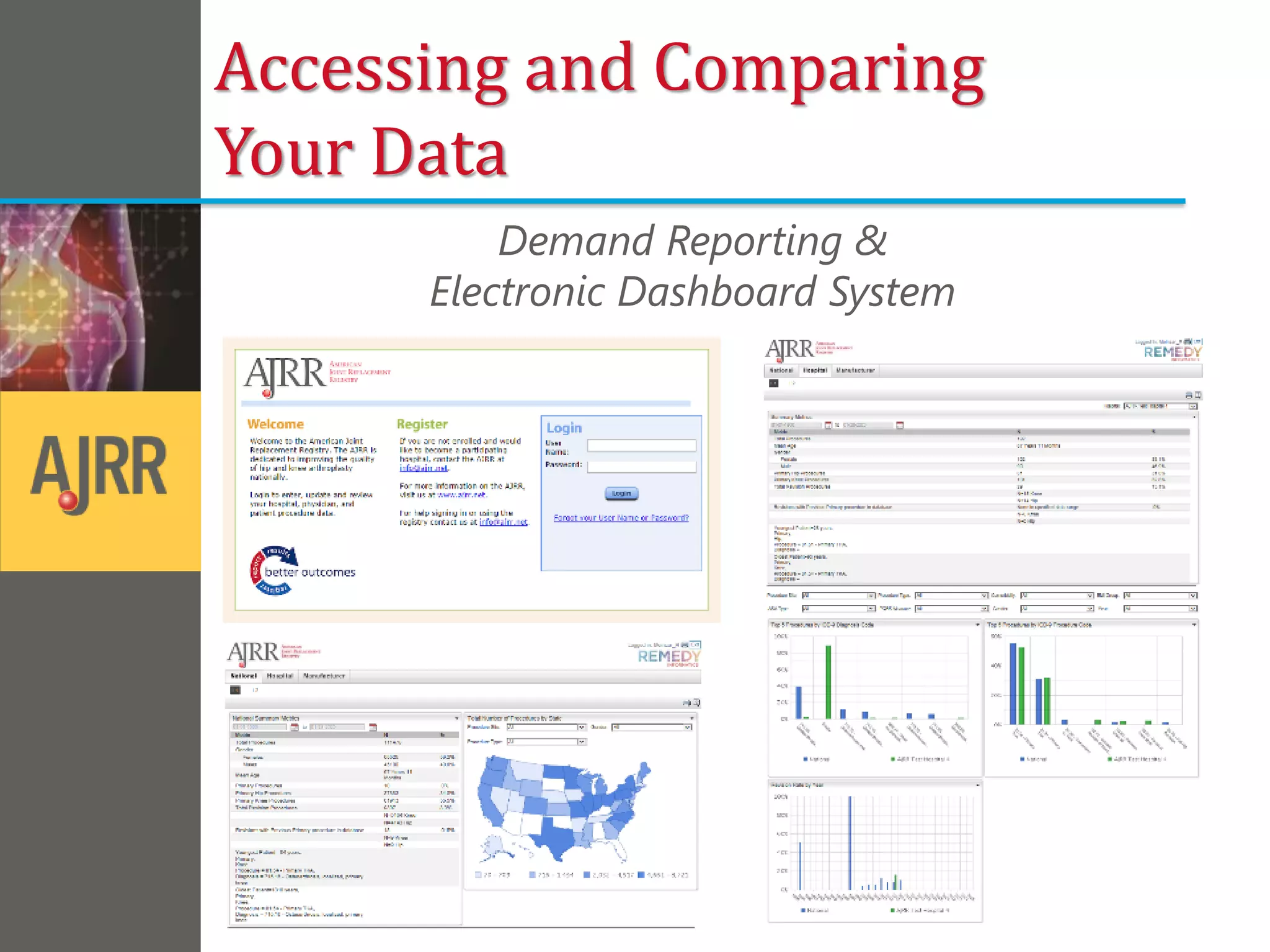
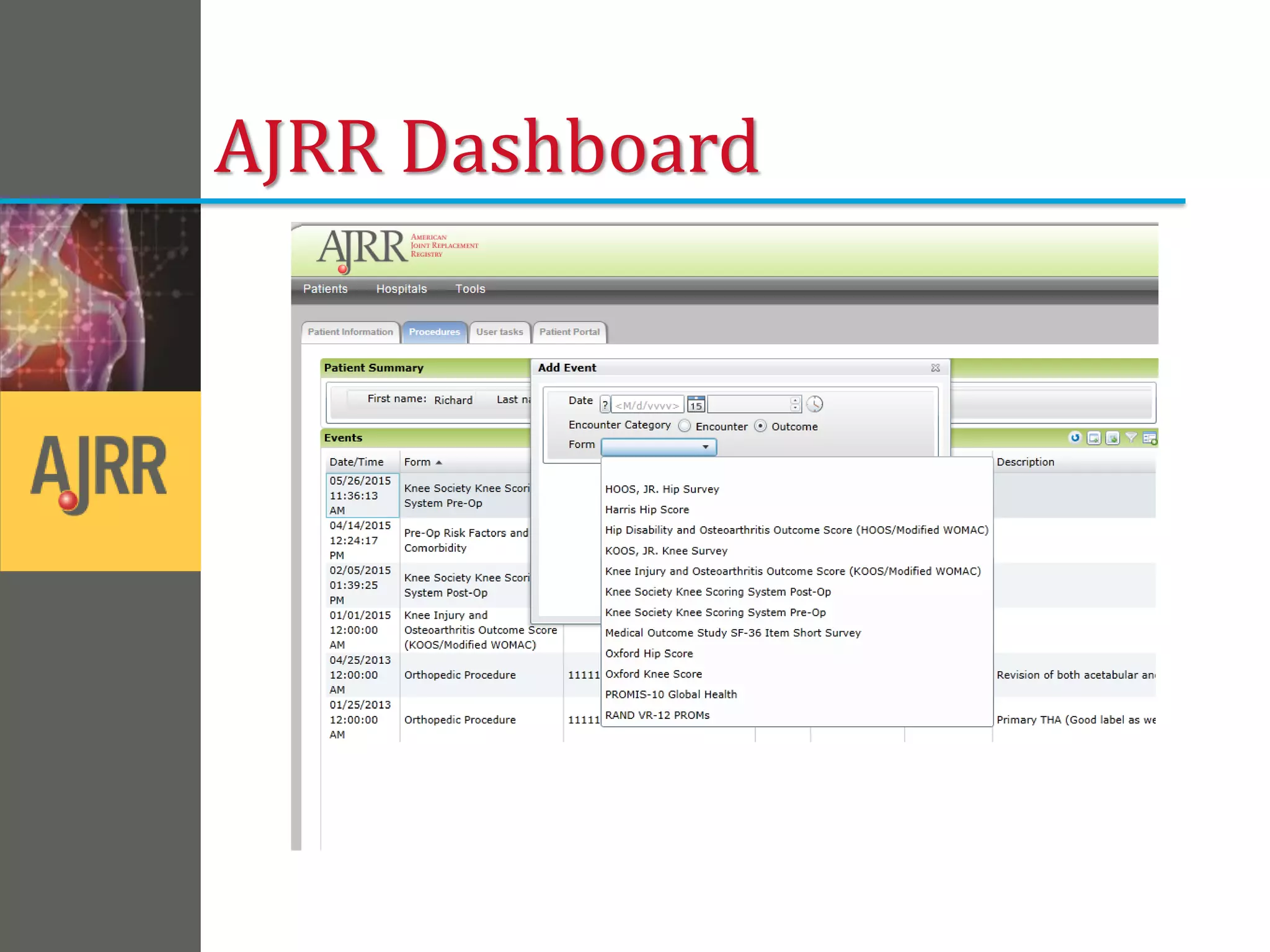
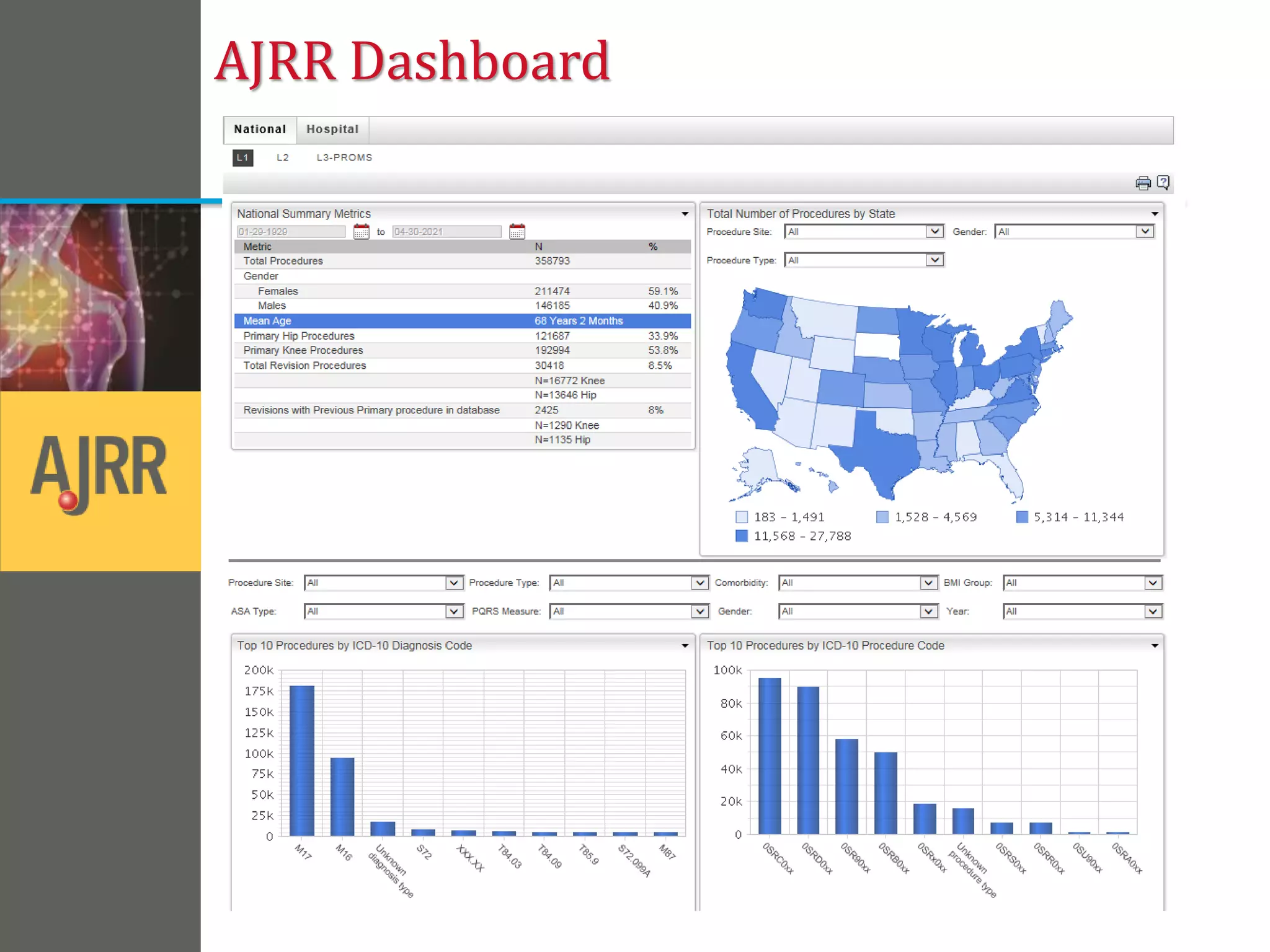
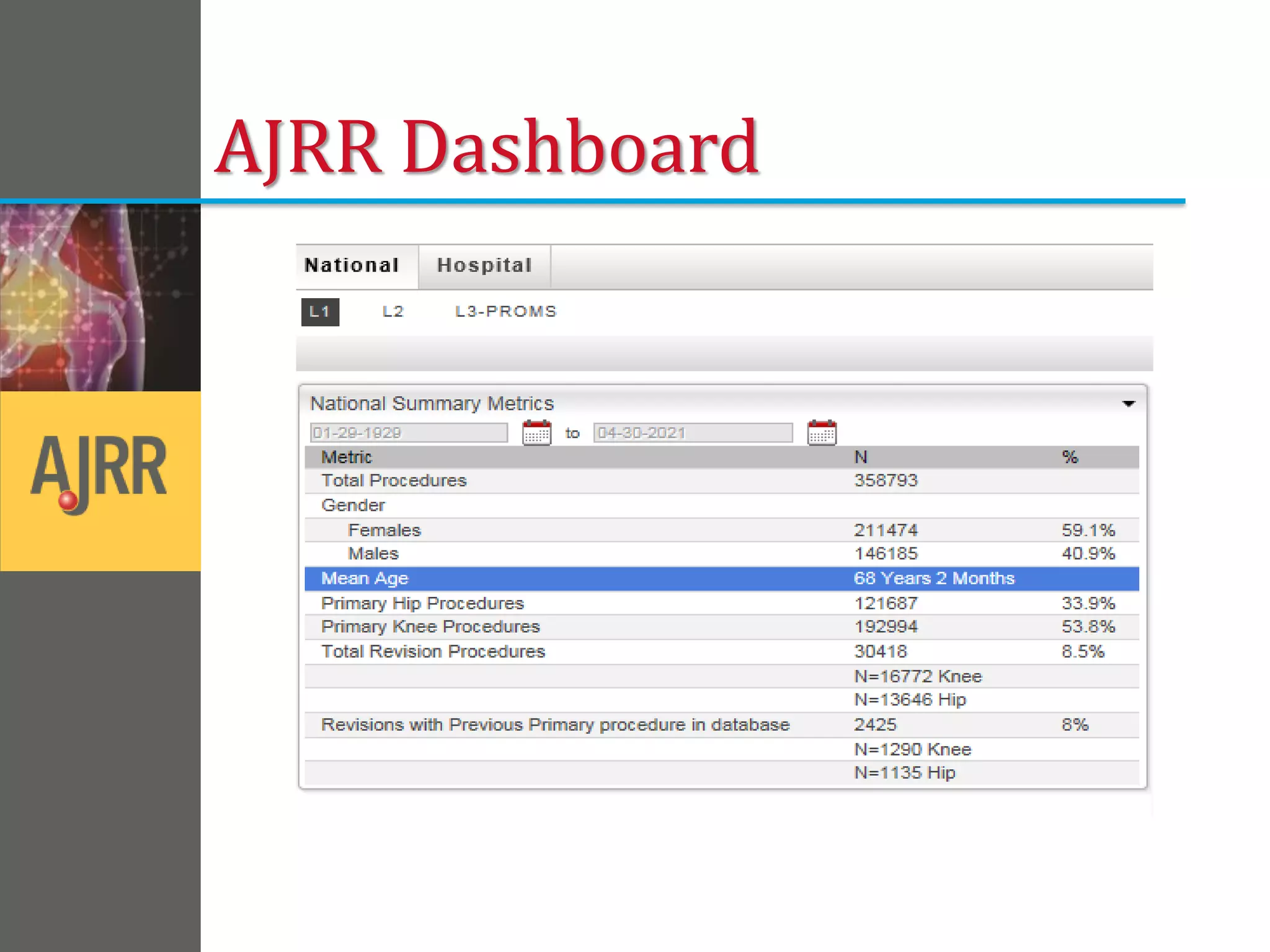
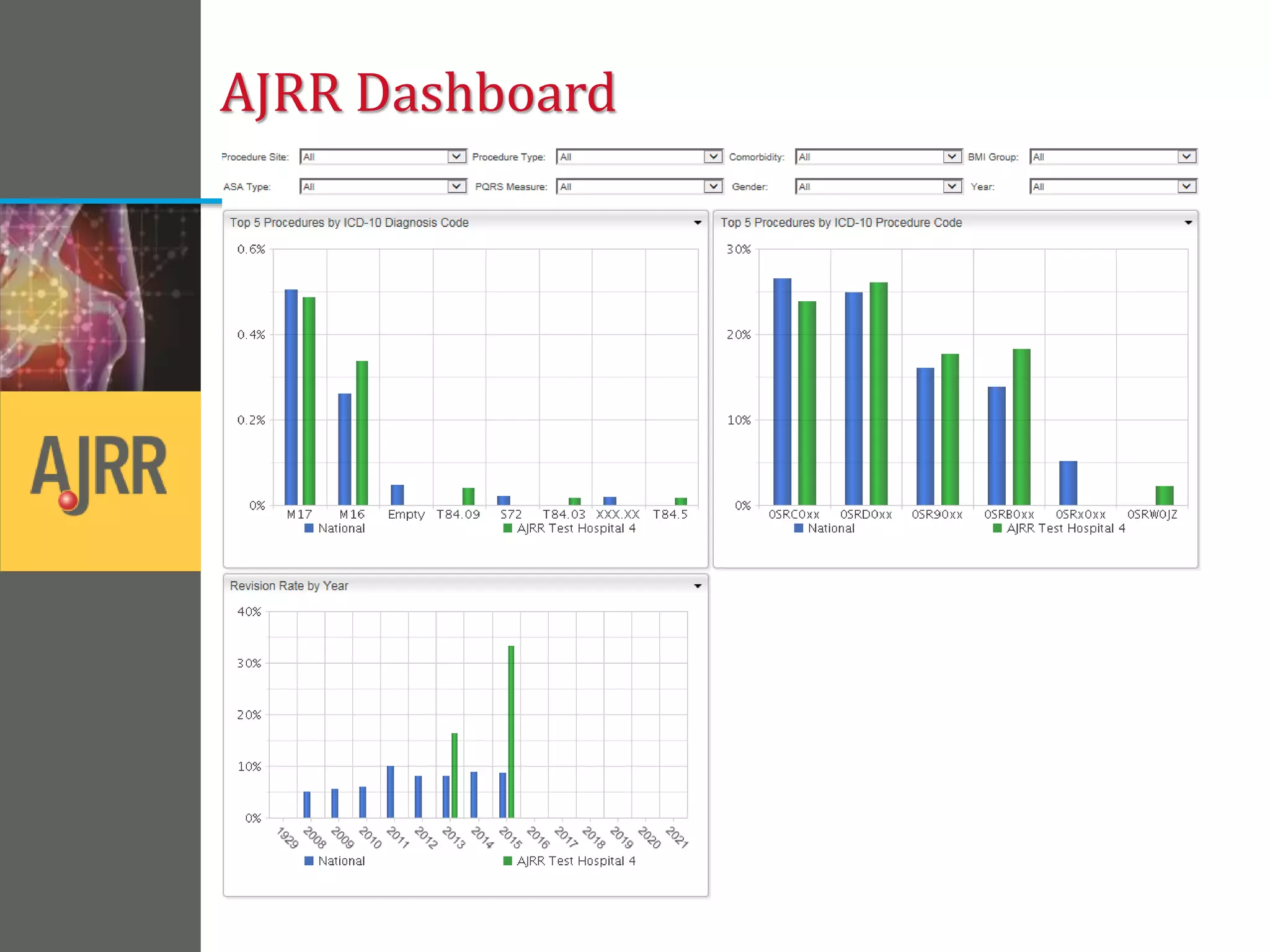
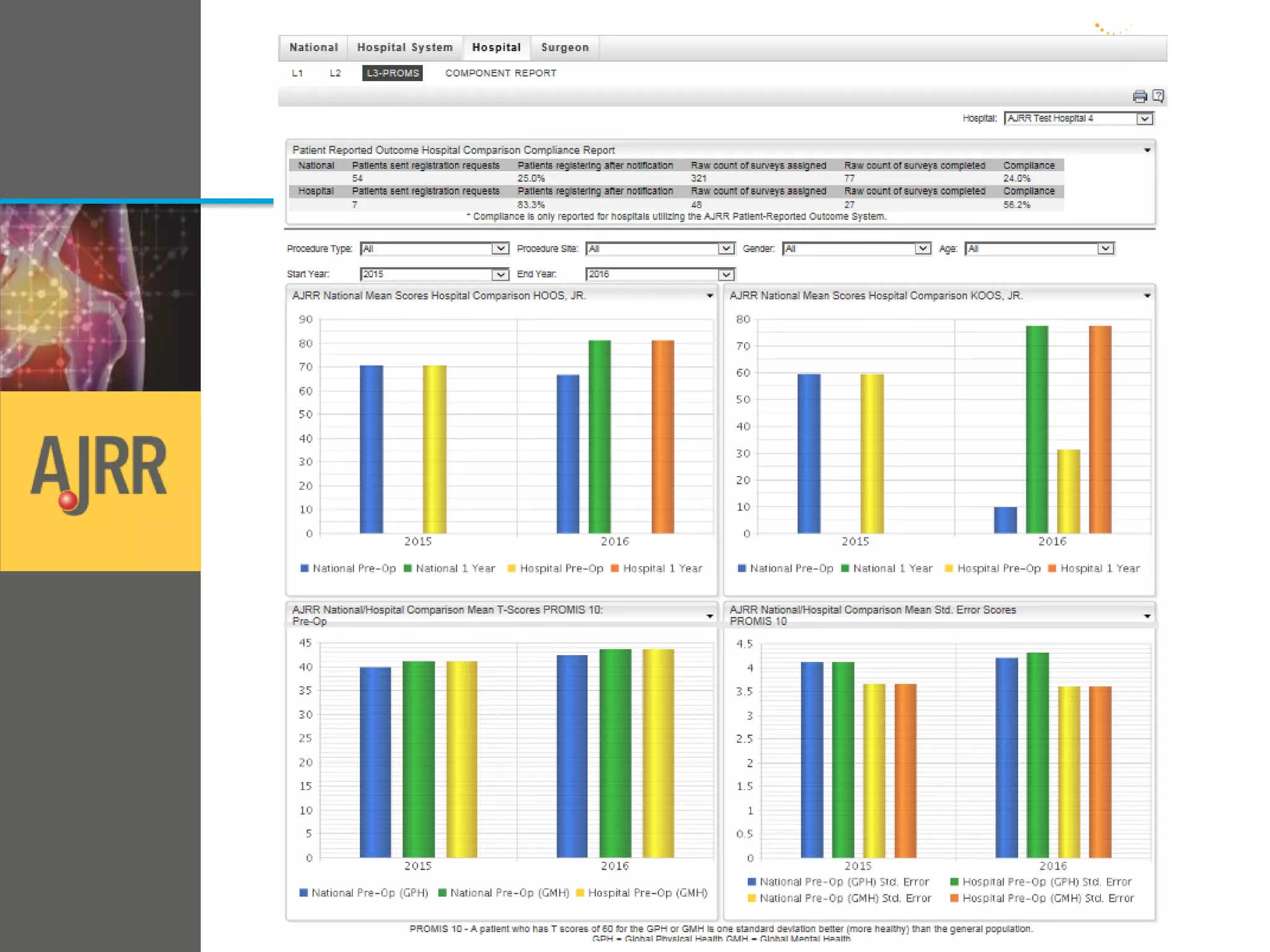
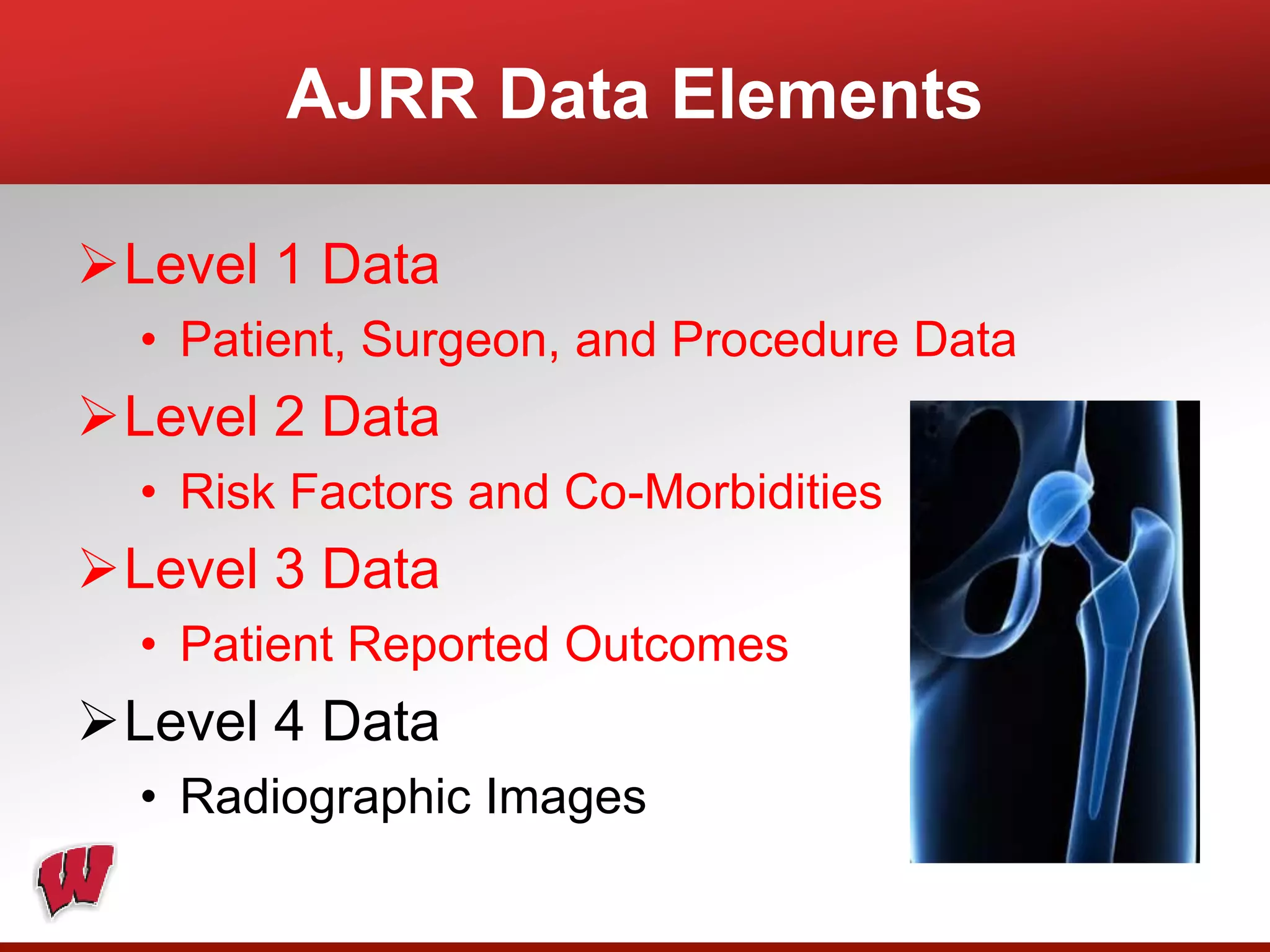
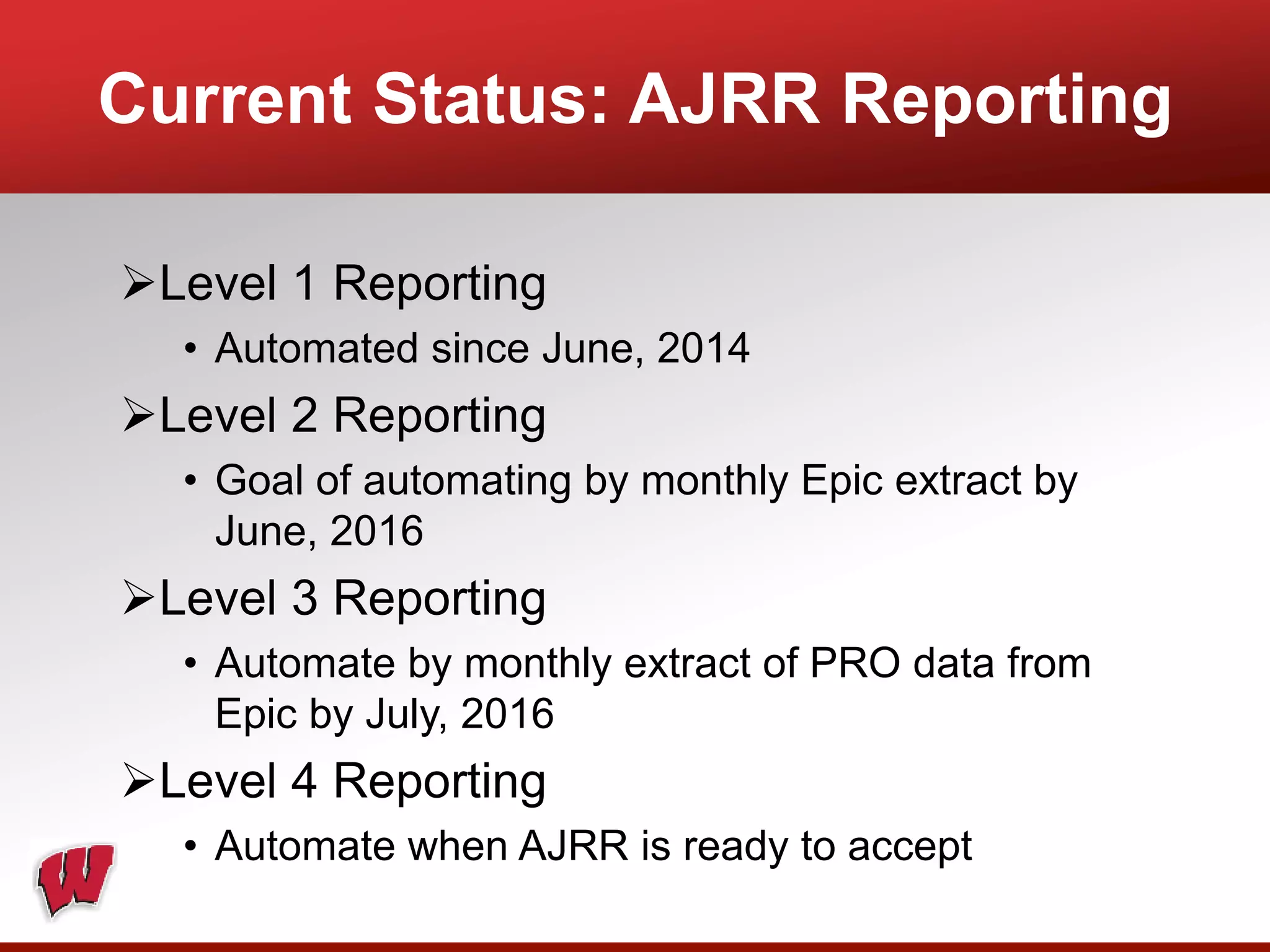
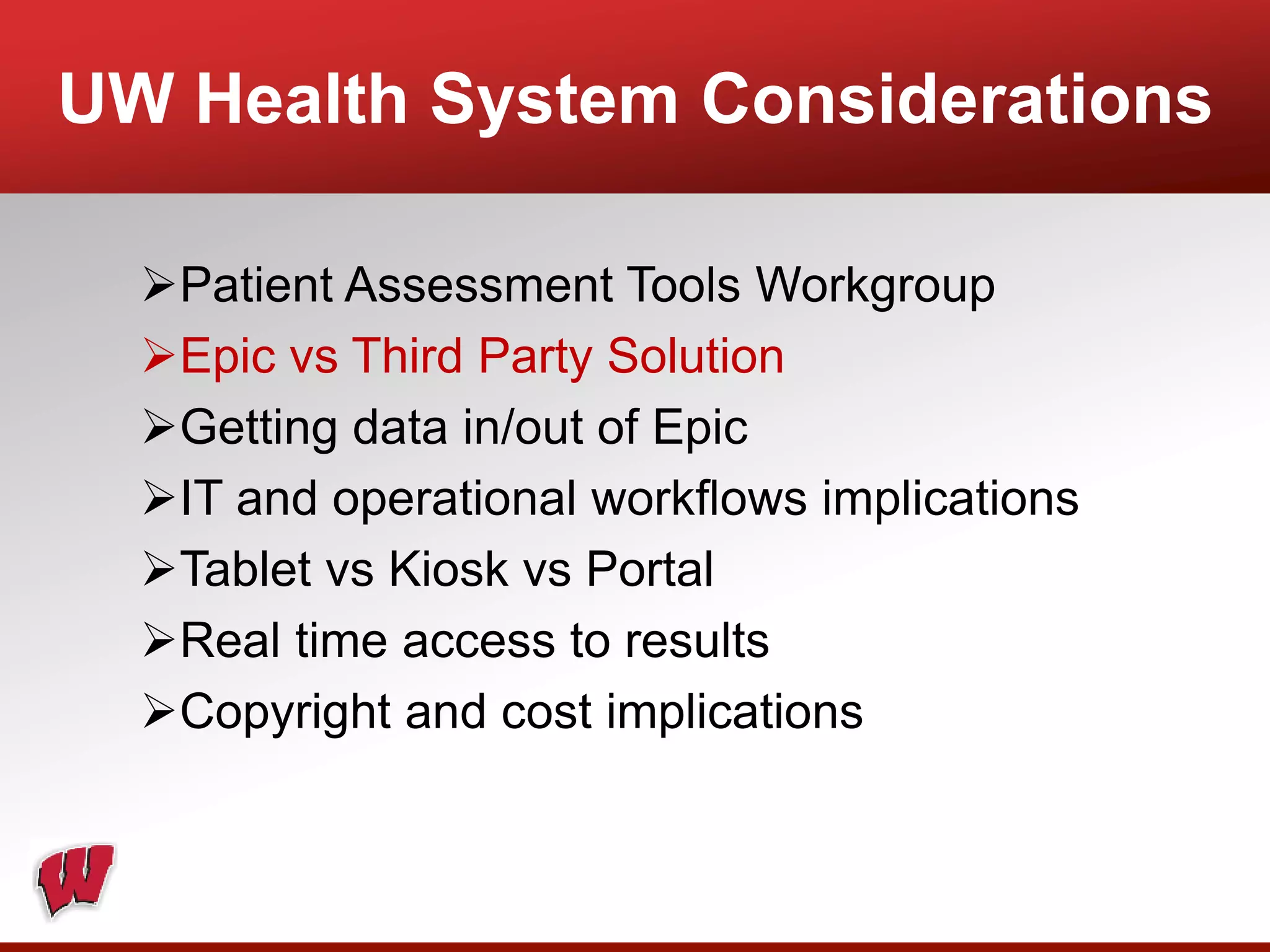
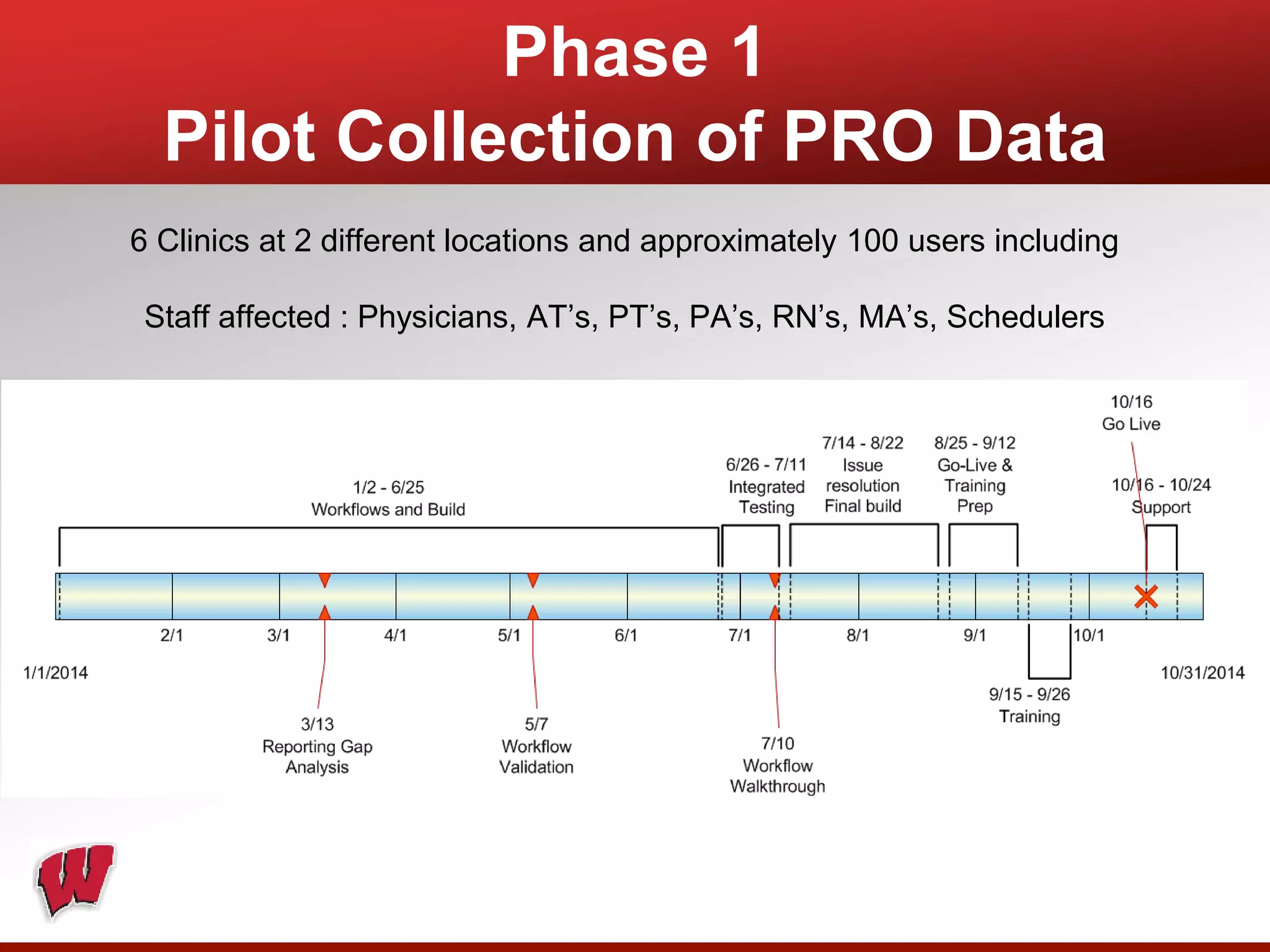
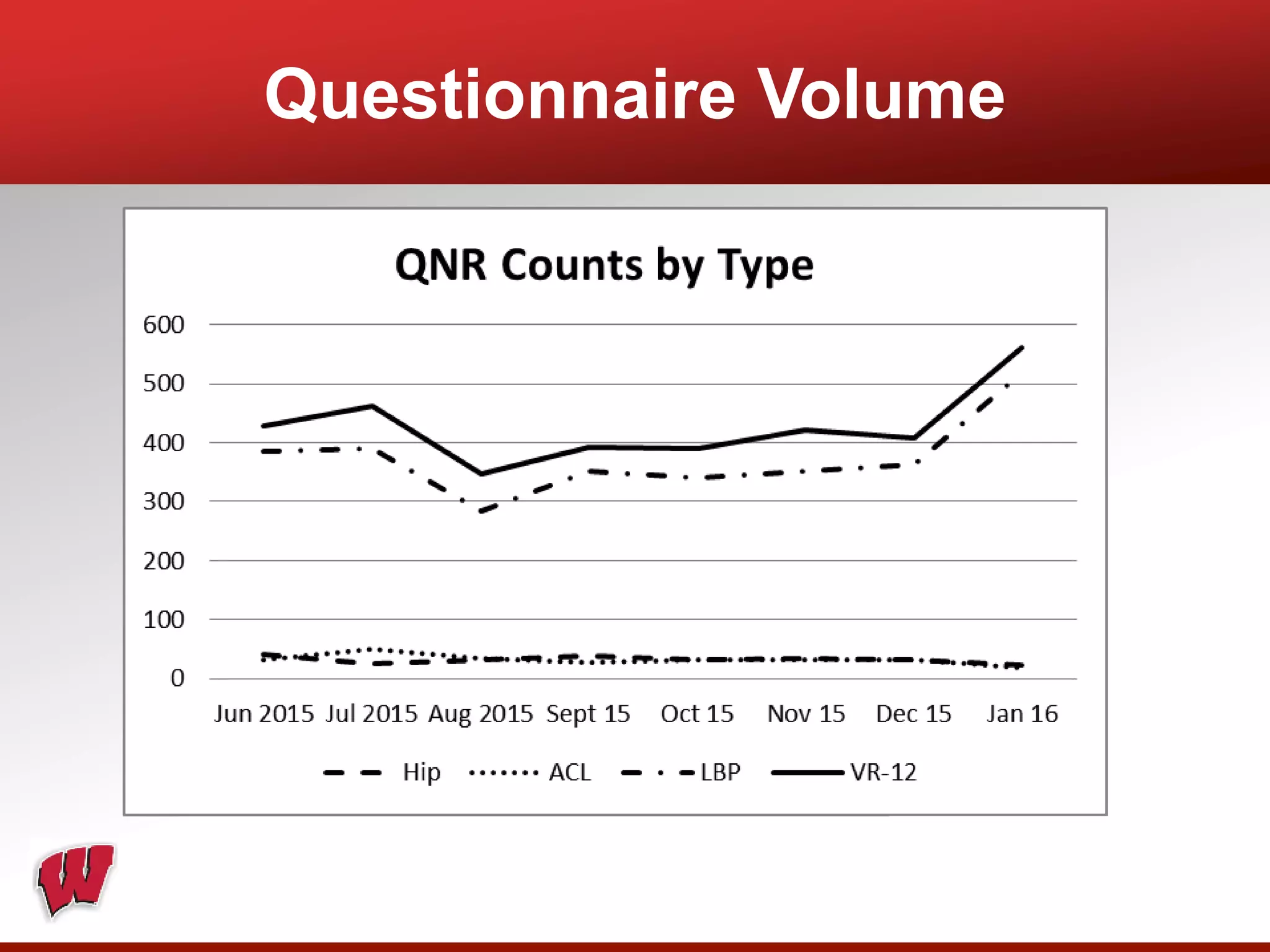
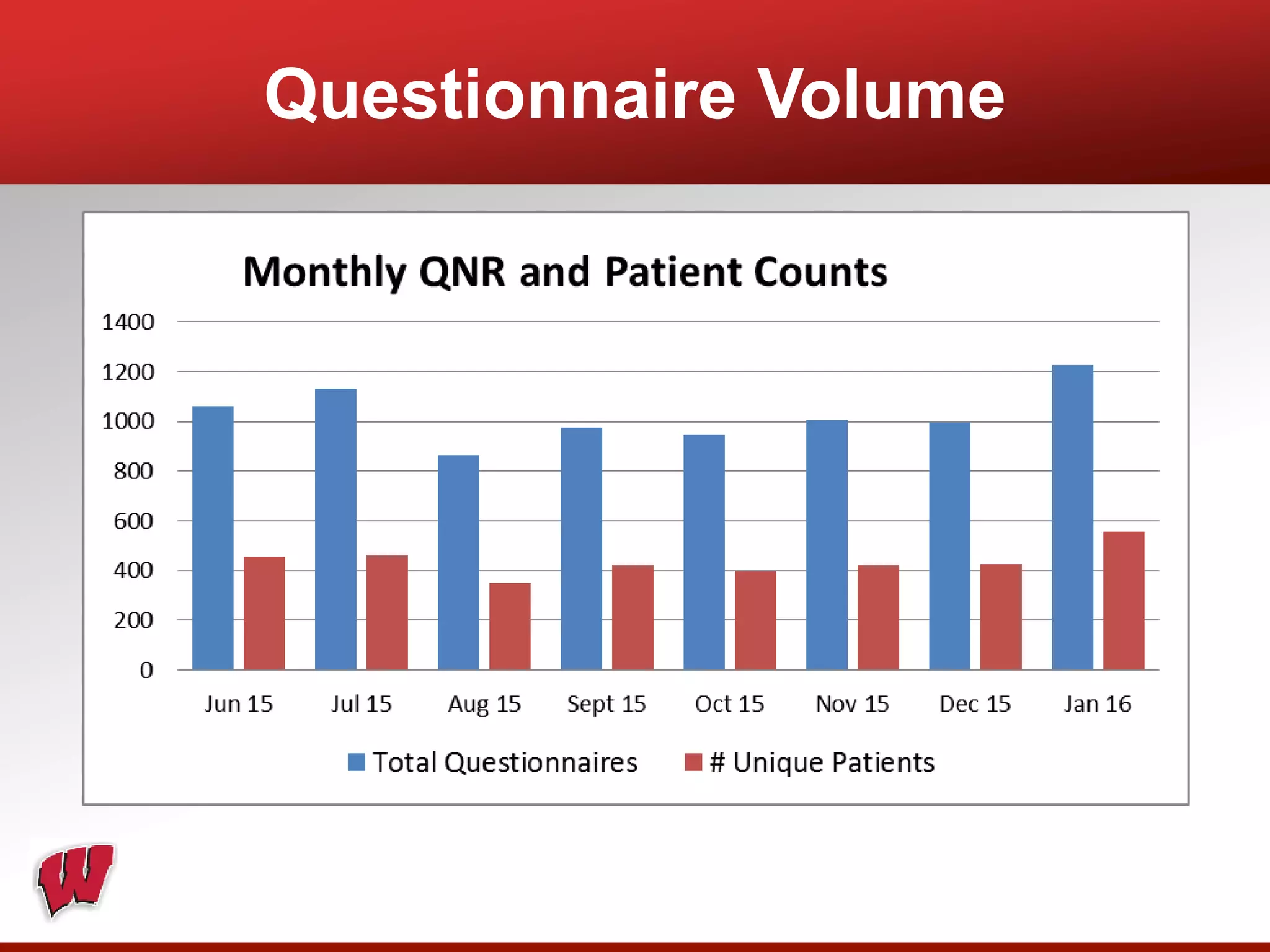
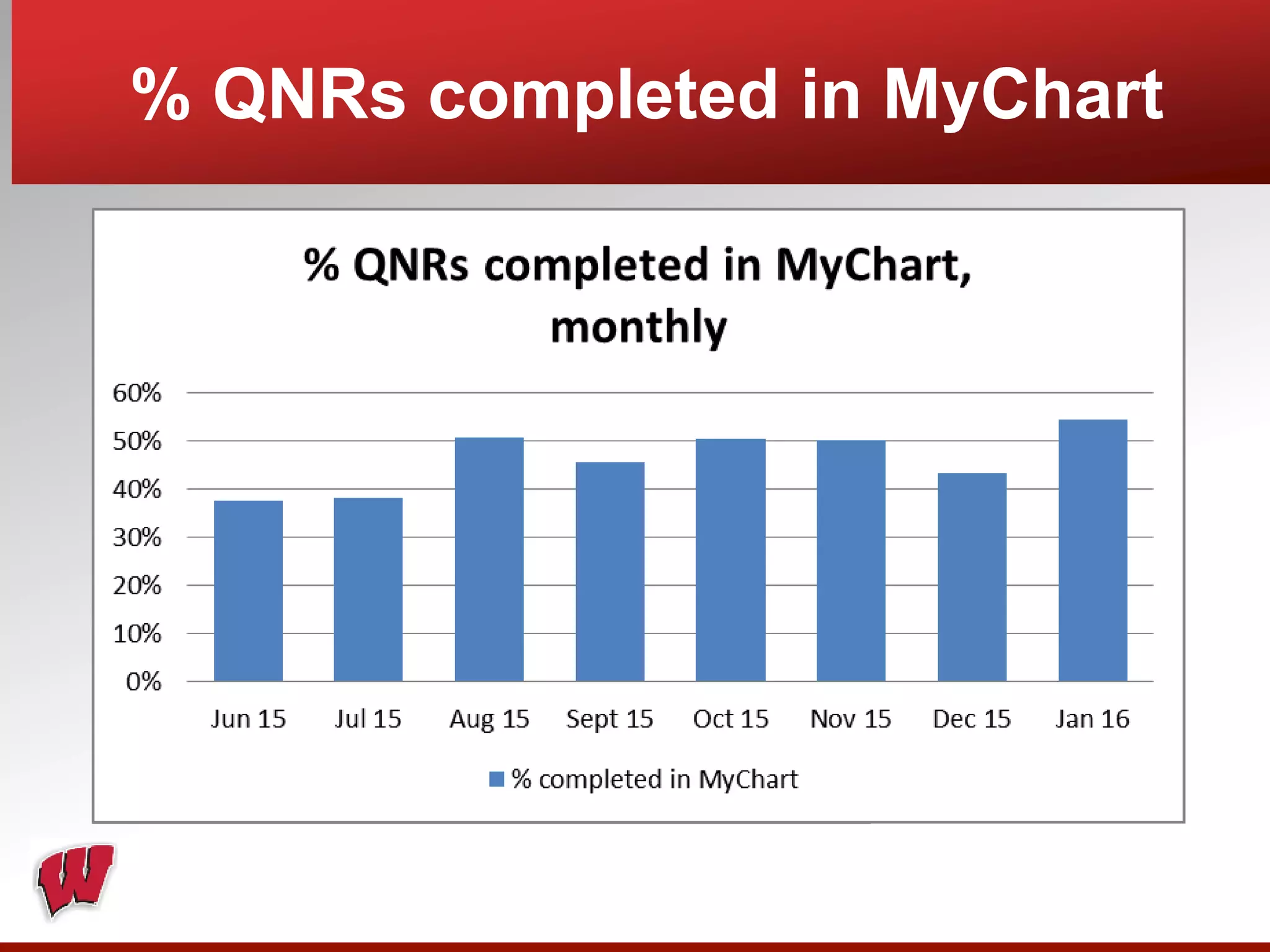
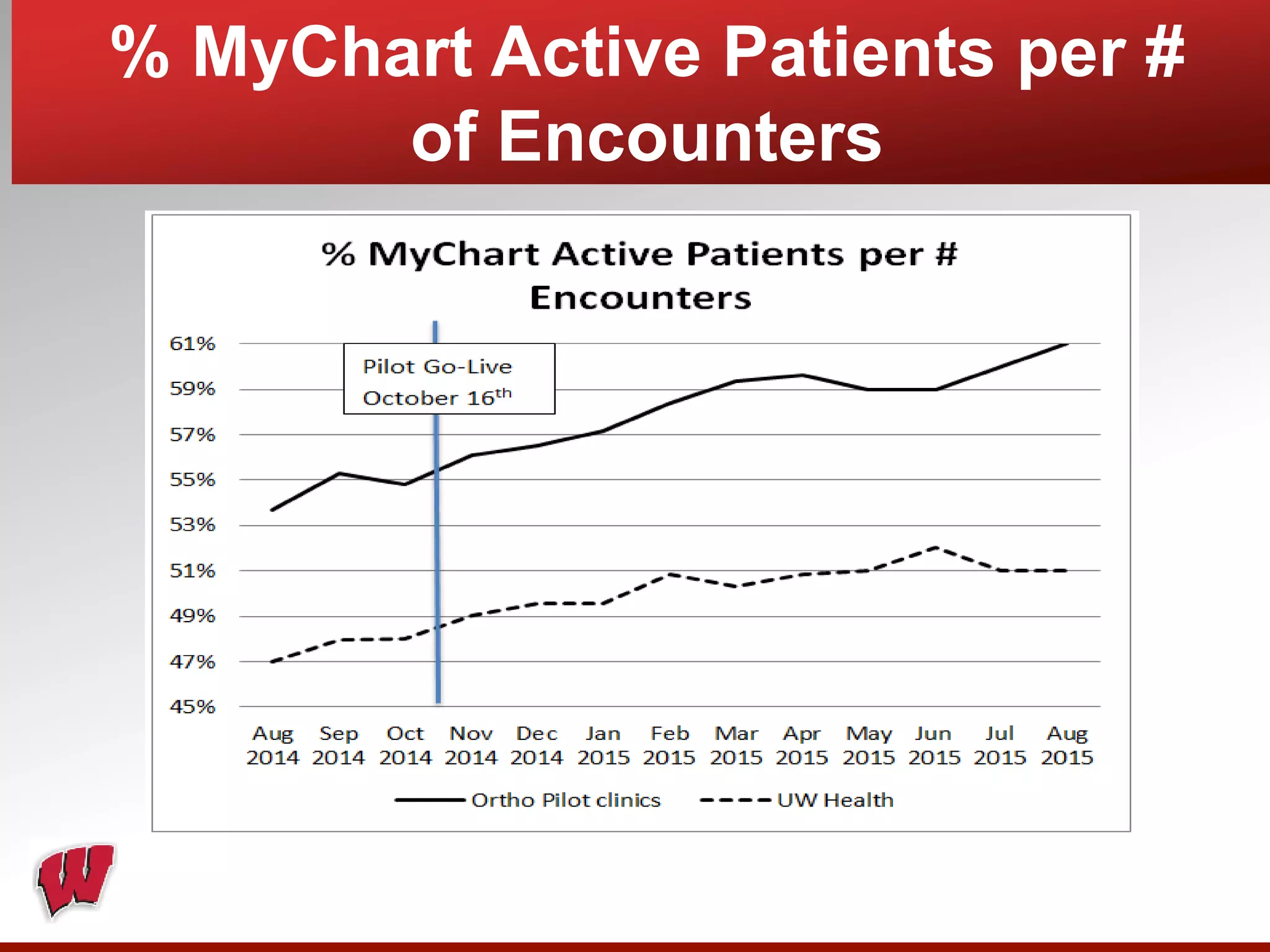
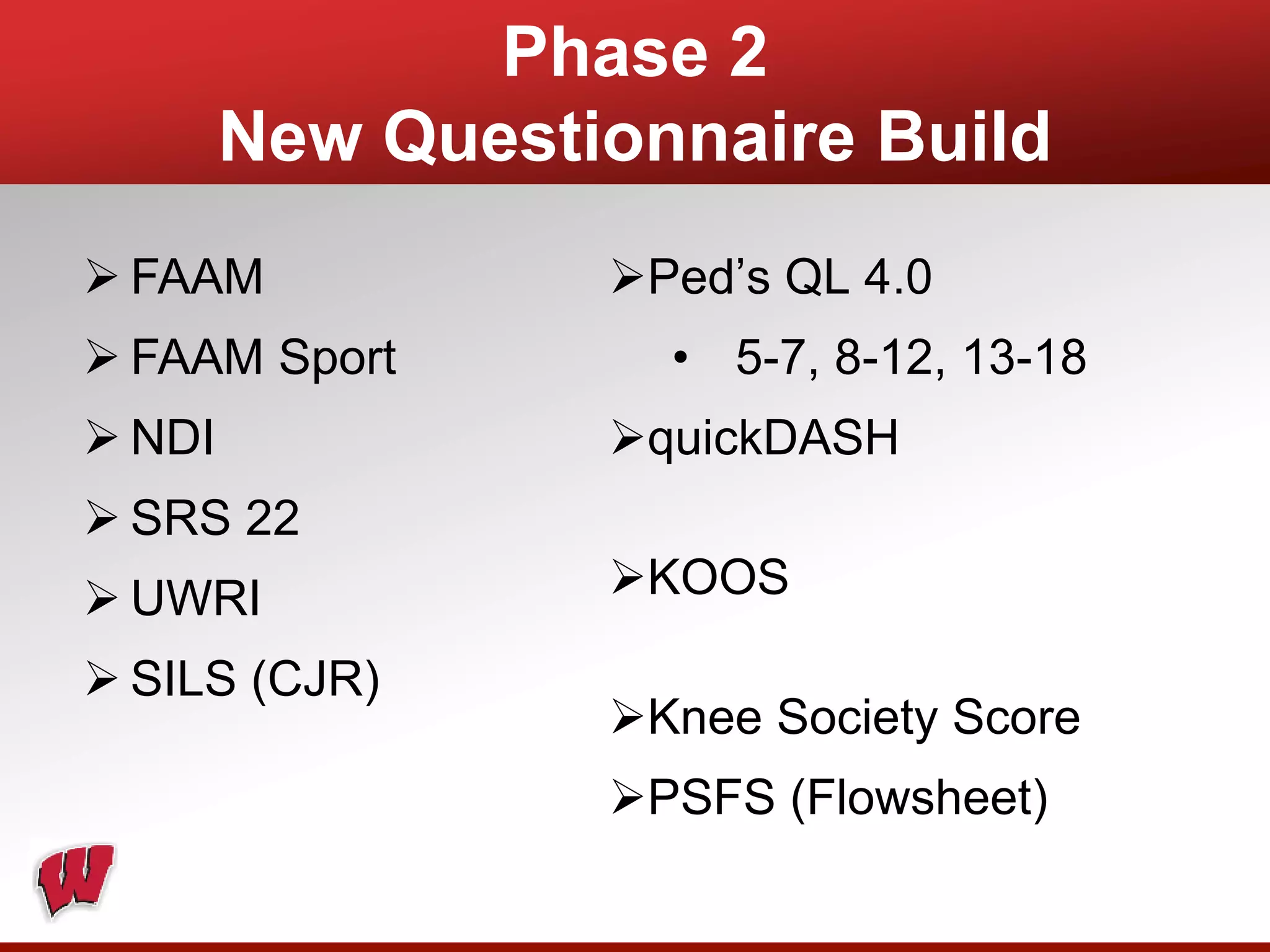
This document provides an overview of registry participation and collecting patient-reported outcome measures through a registry. It discusses the University of Wisconsin's process for collecting PROs in their orthopedic clinics in two phases: a pilot phase and a full implementation phase. The pilot involved collecting PROs in 6 clinics using Epic and tablet computers. Lessons learned included that an integrated tablet/portal solution and coordinated project management were important. The full implementation will expand PRO collection to all orthopedic locations and improve reporting automation.