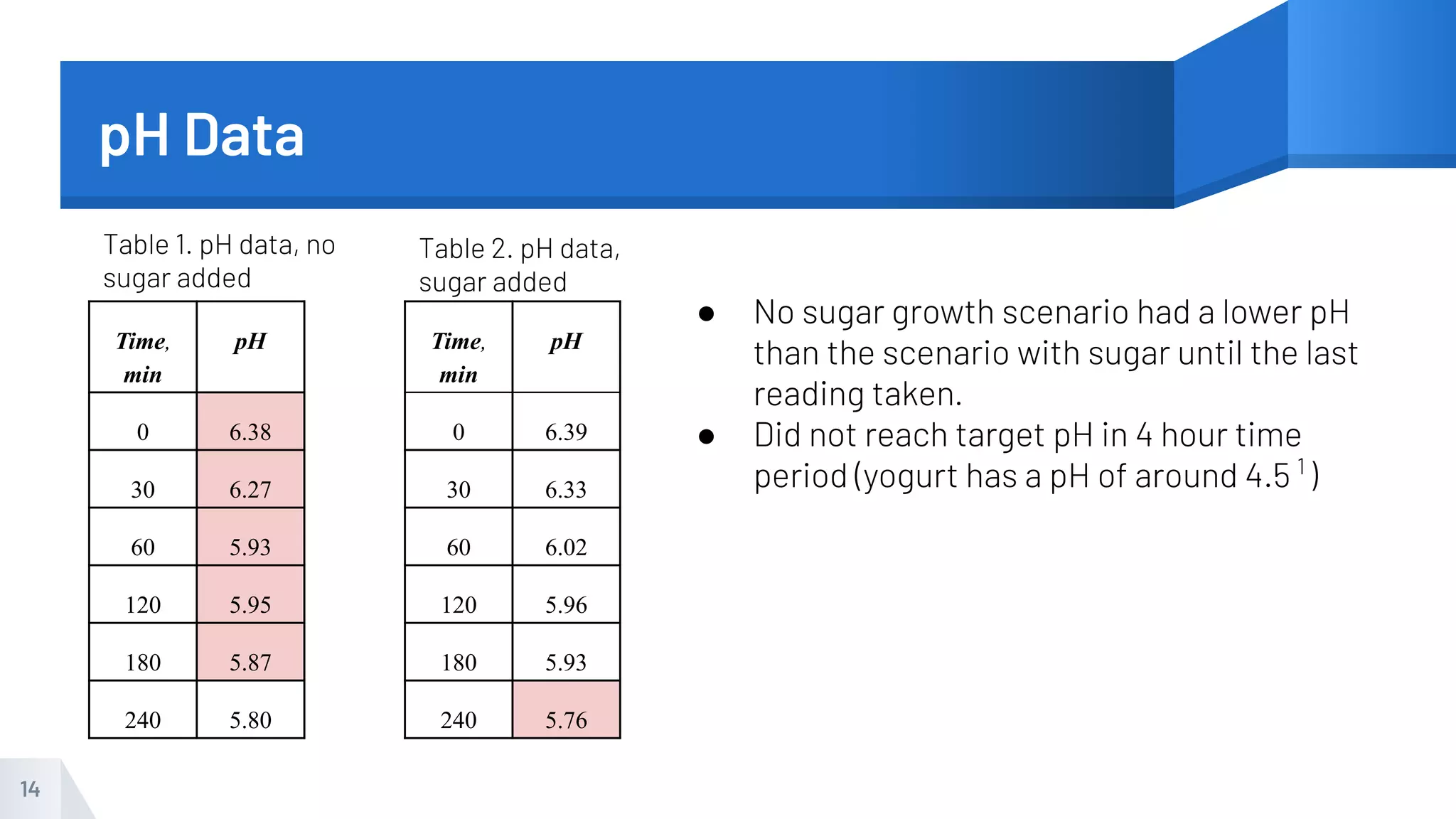
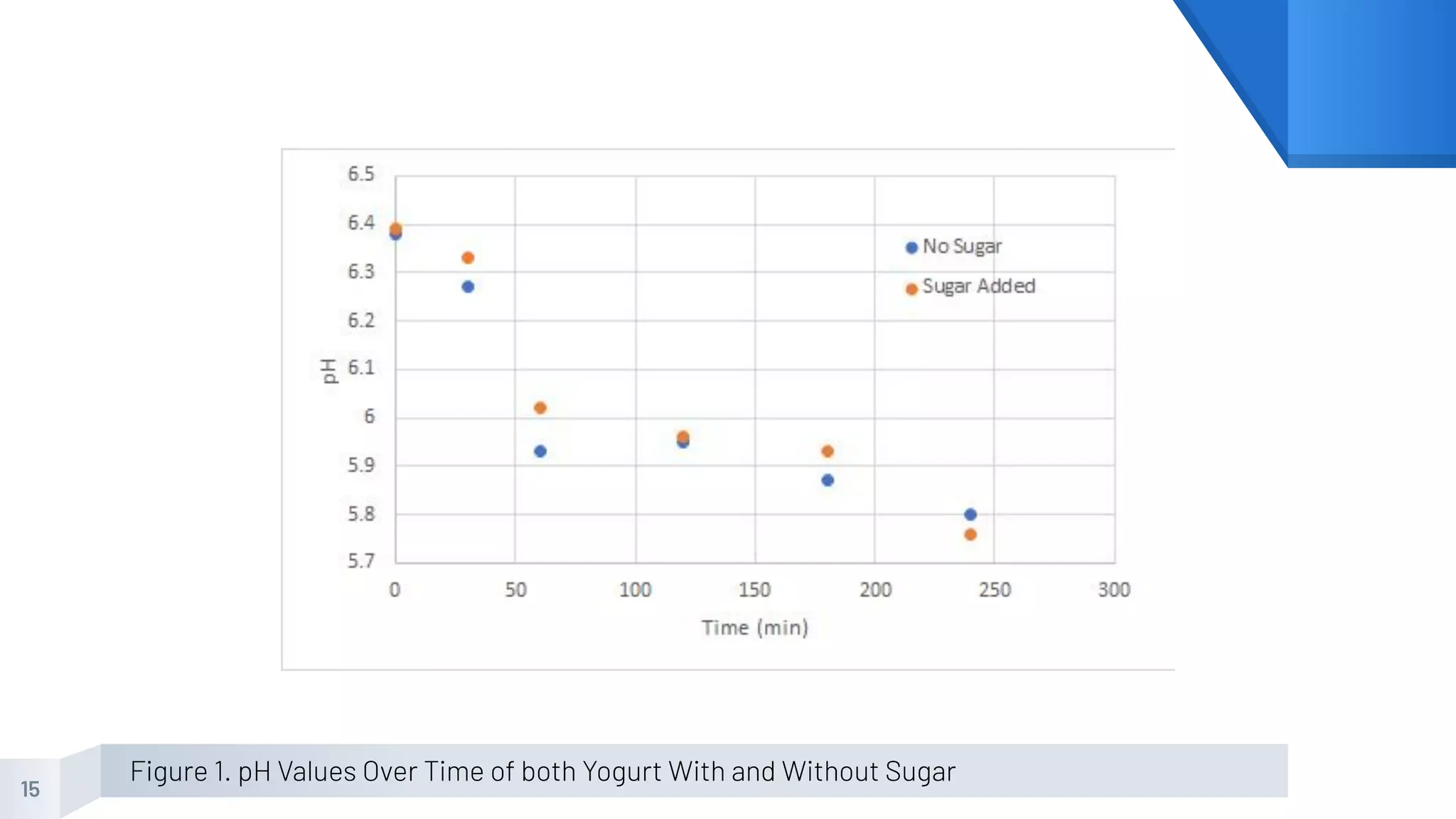
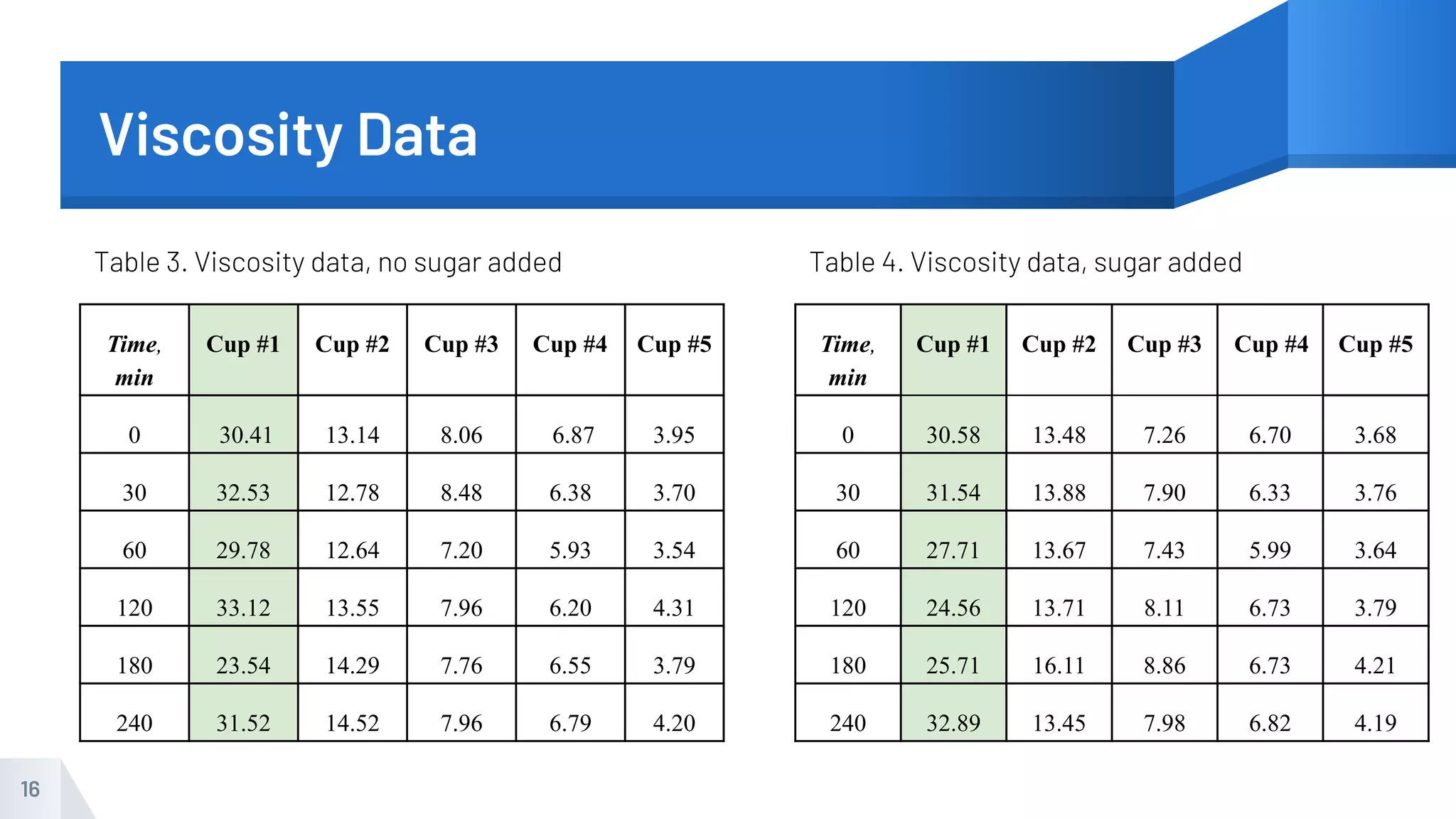
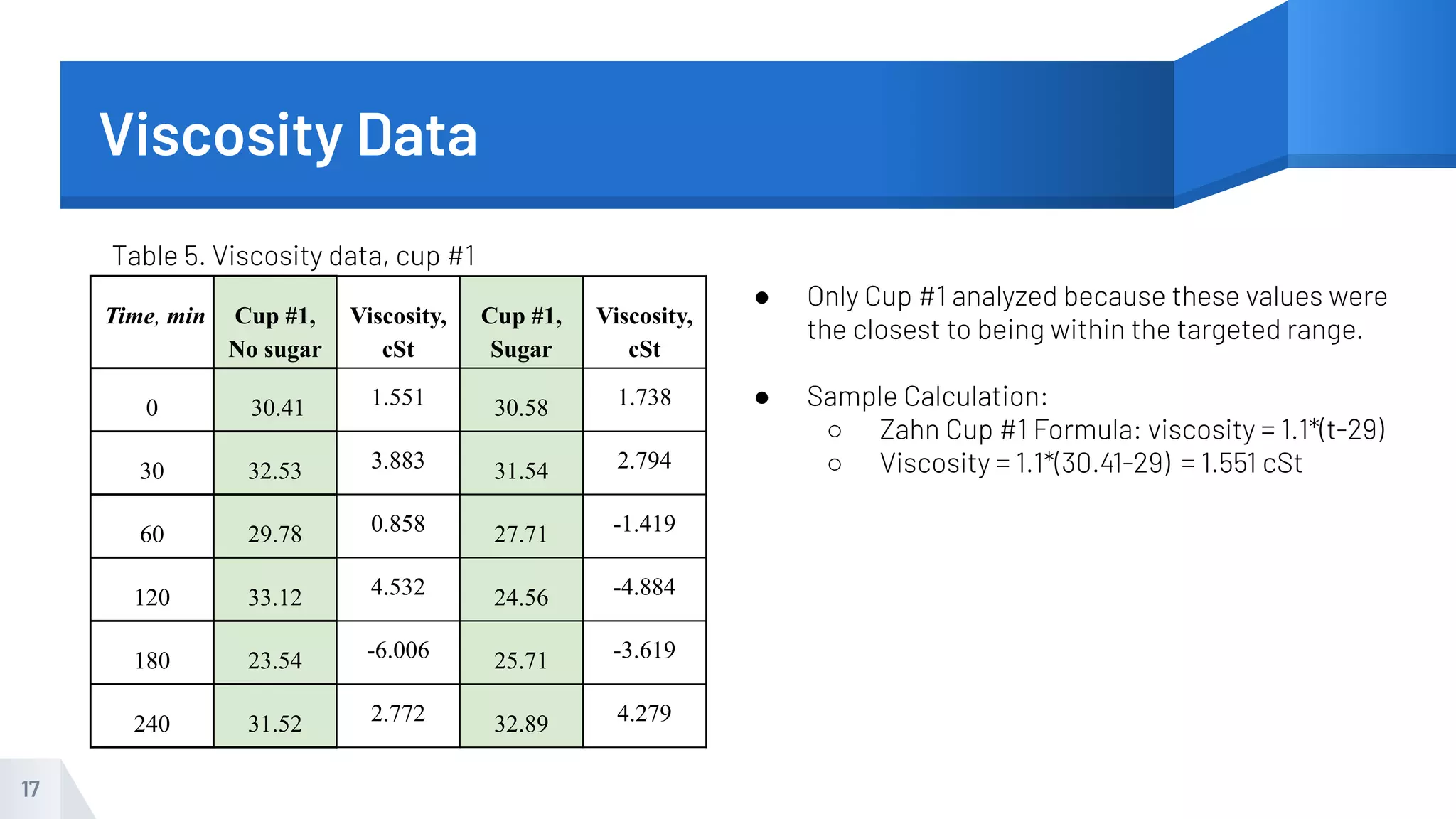
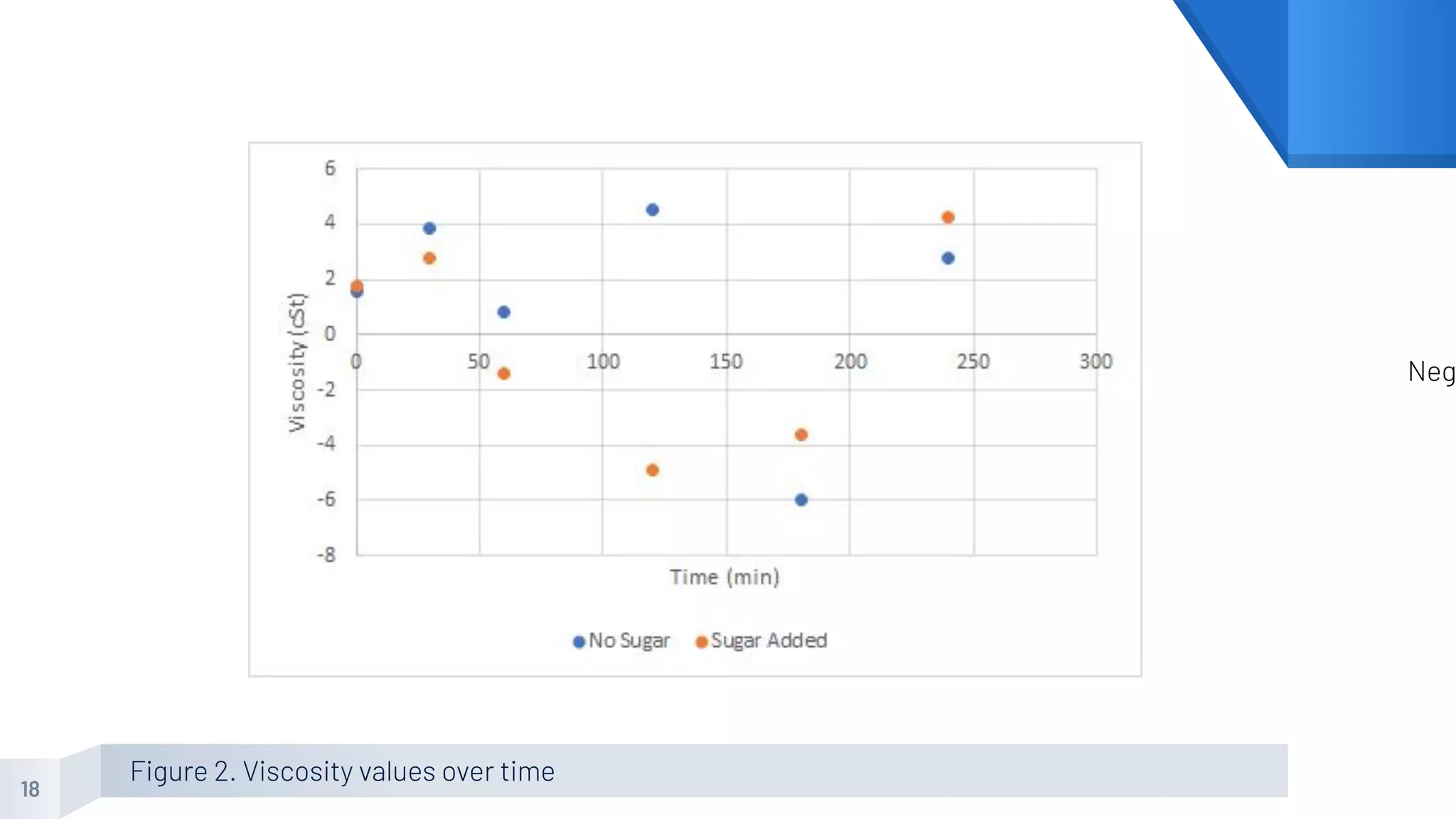
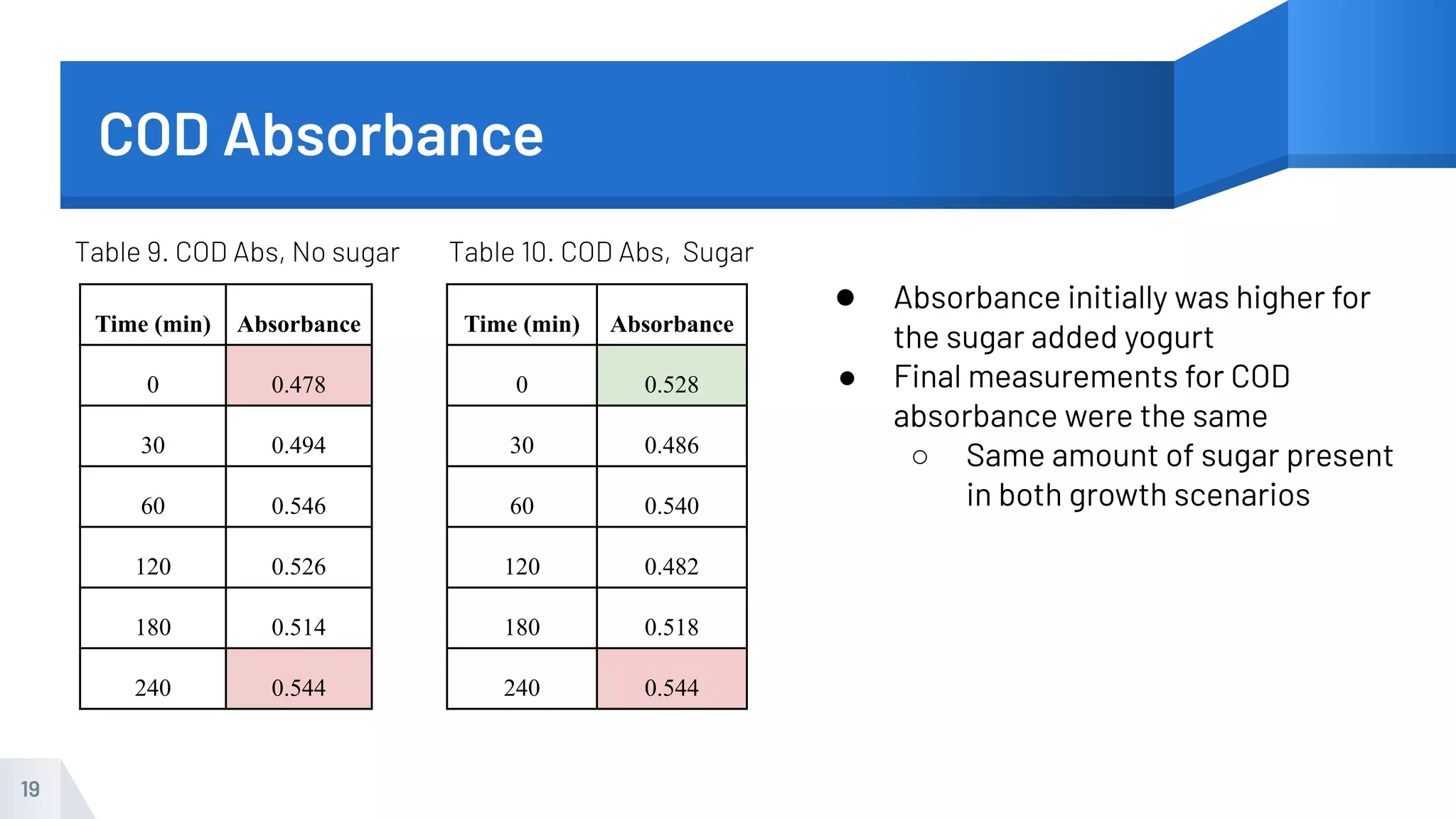
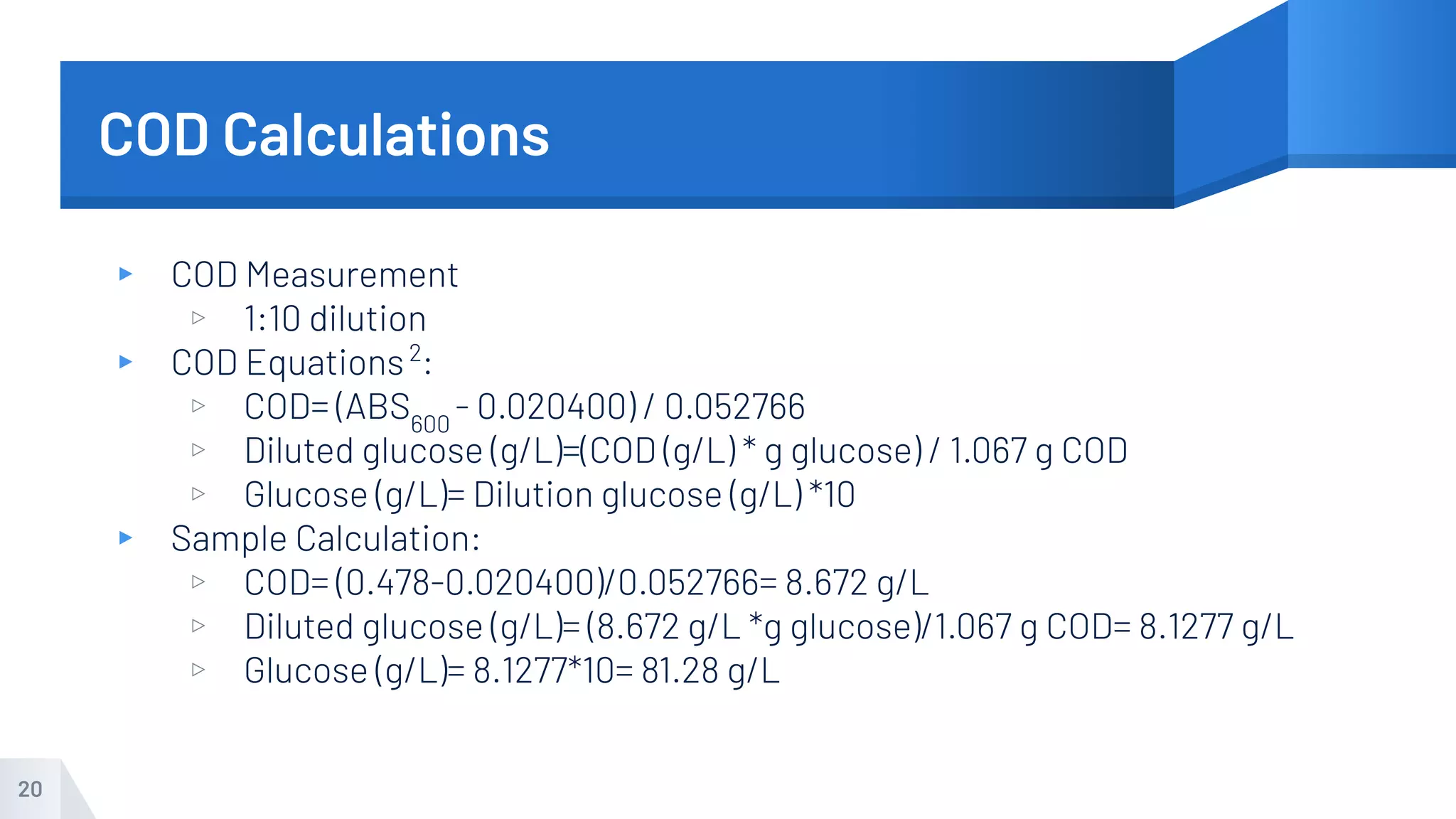
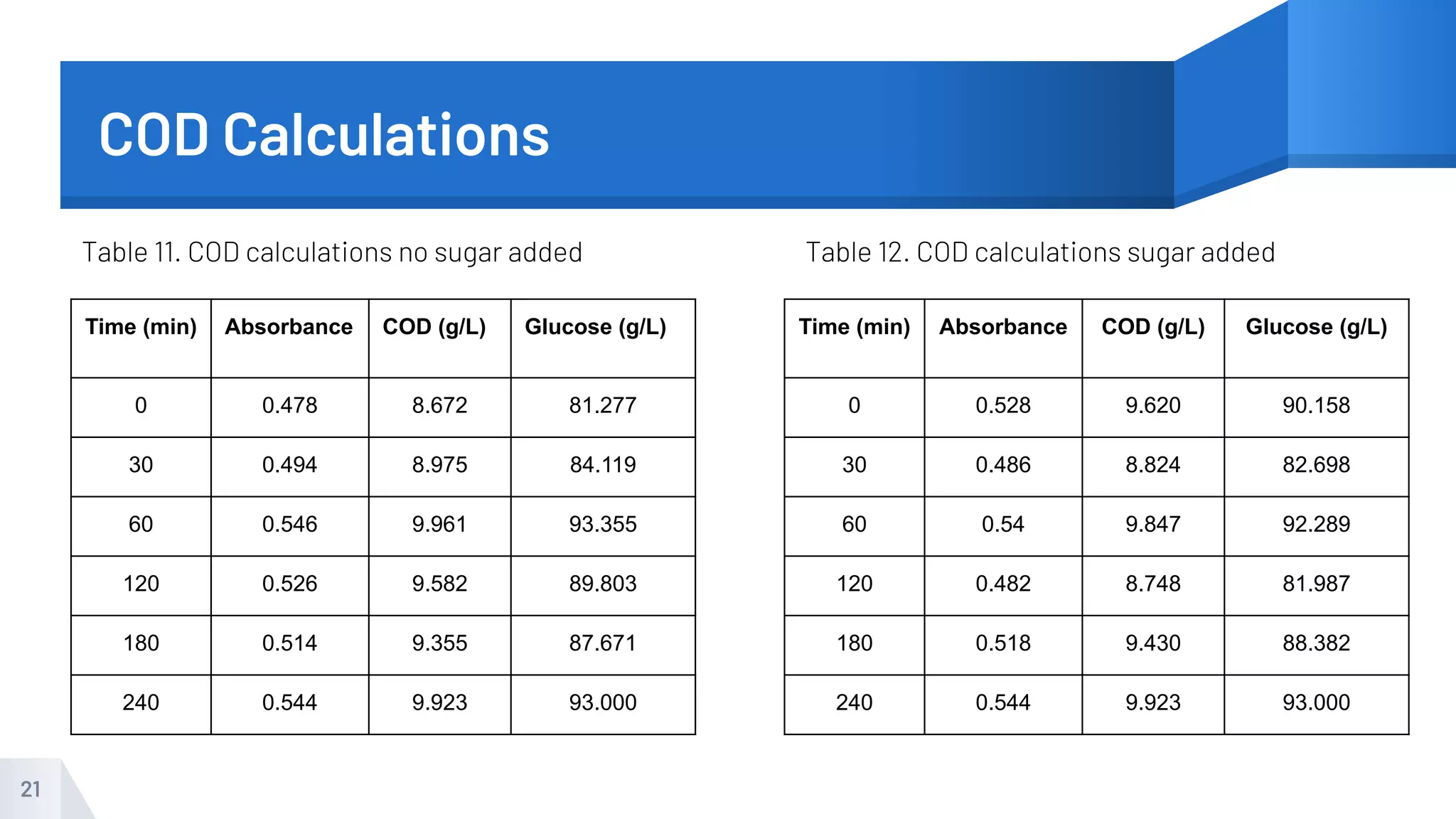
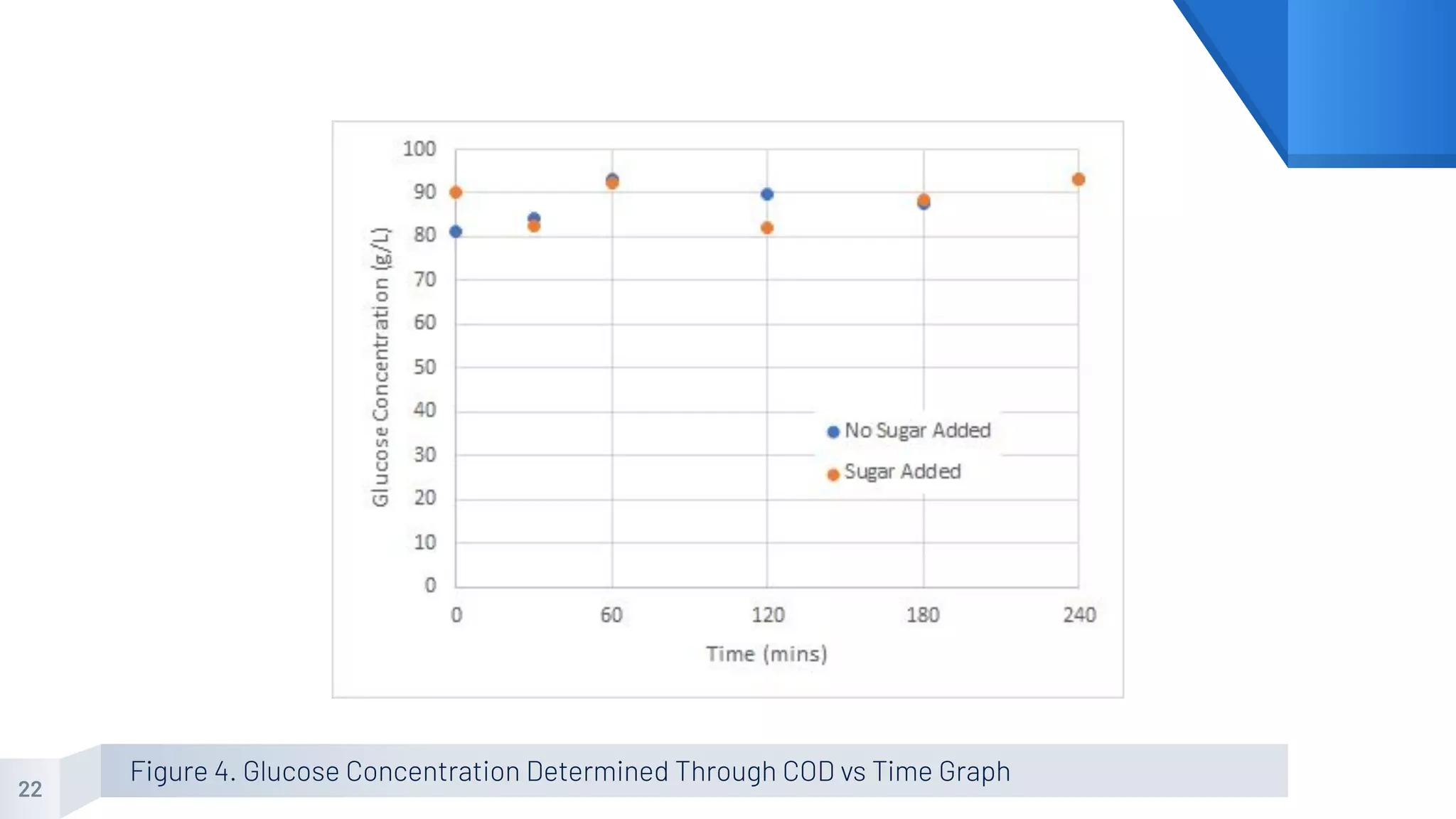
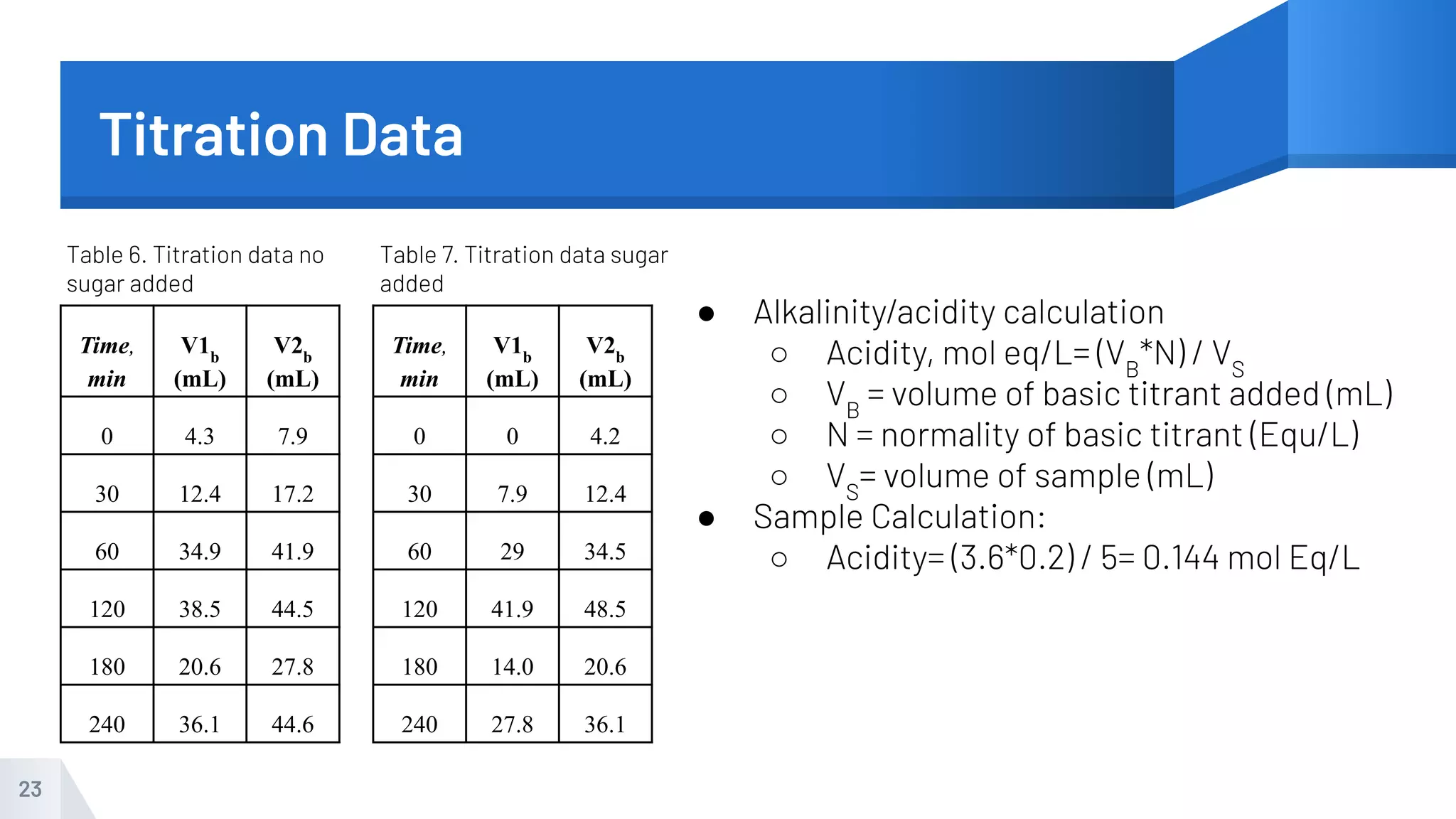
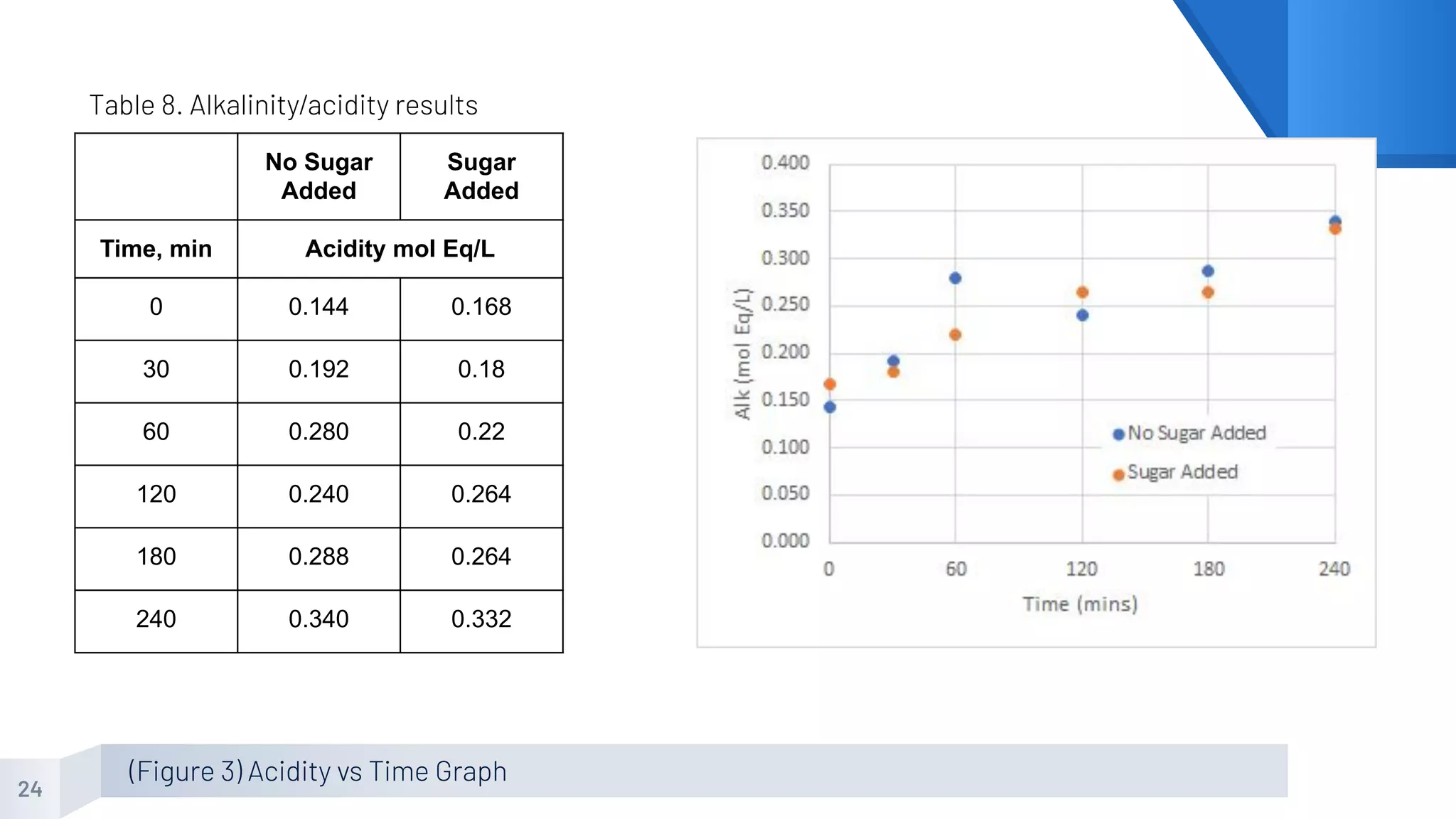
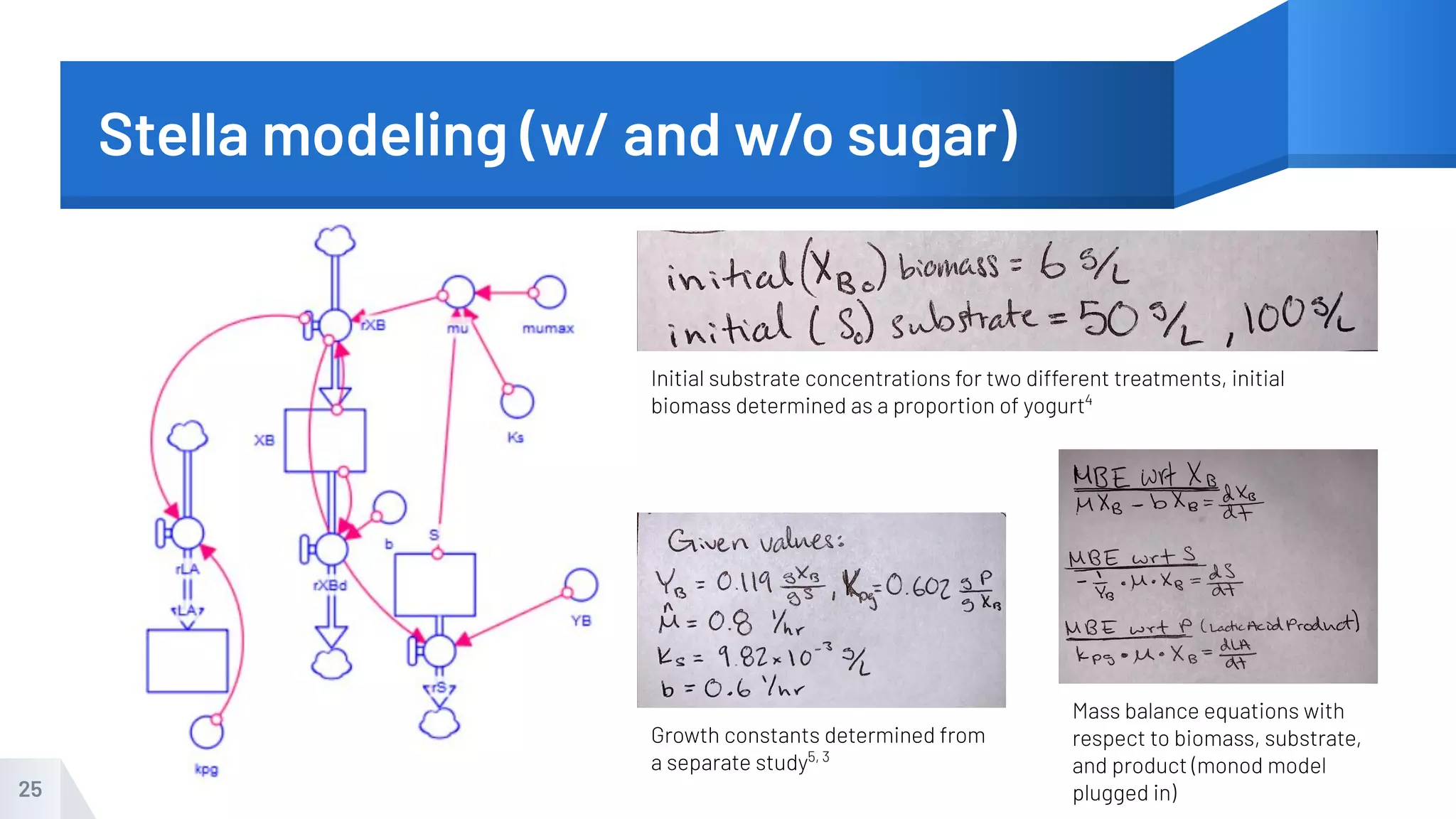
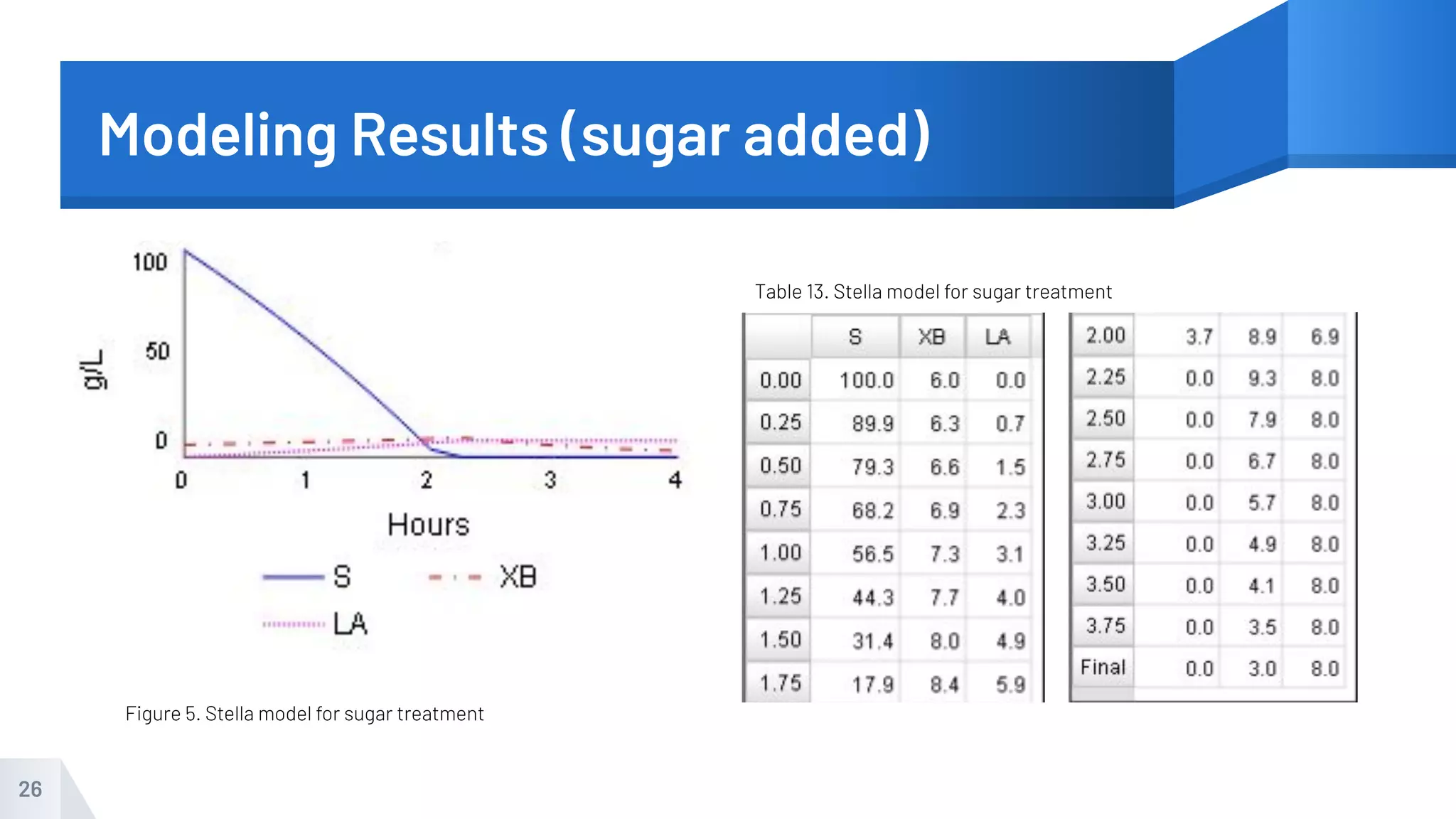
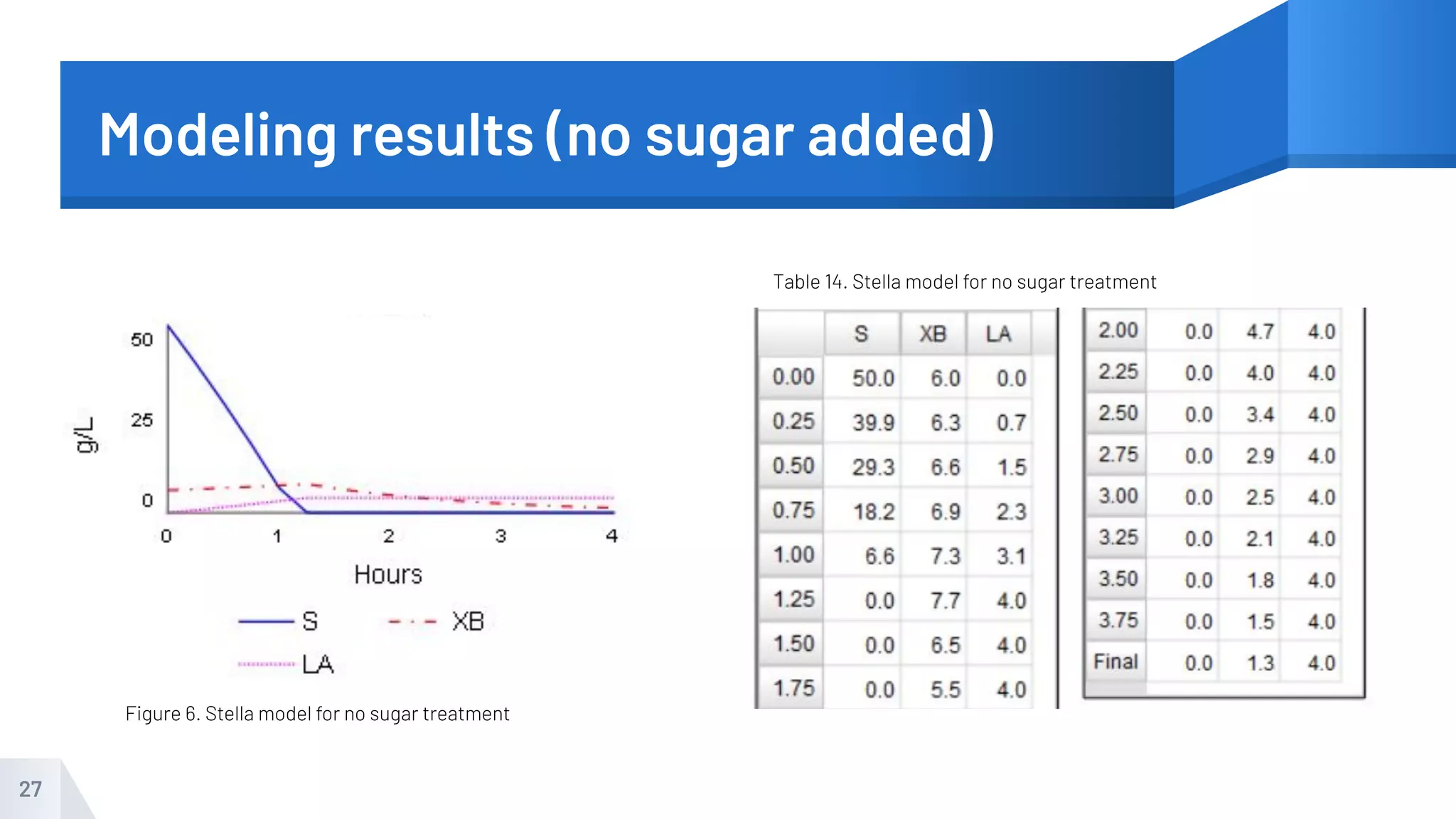
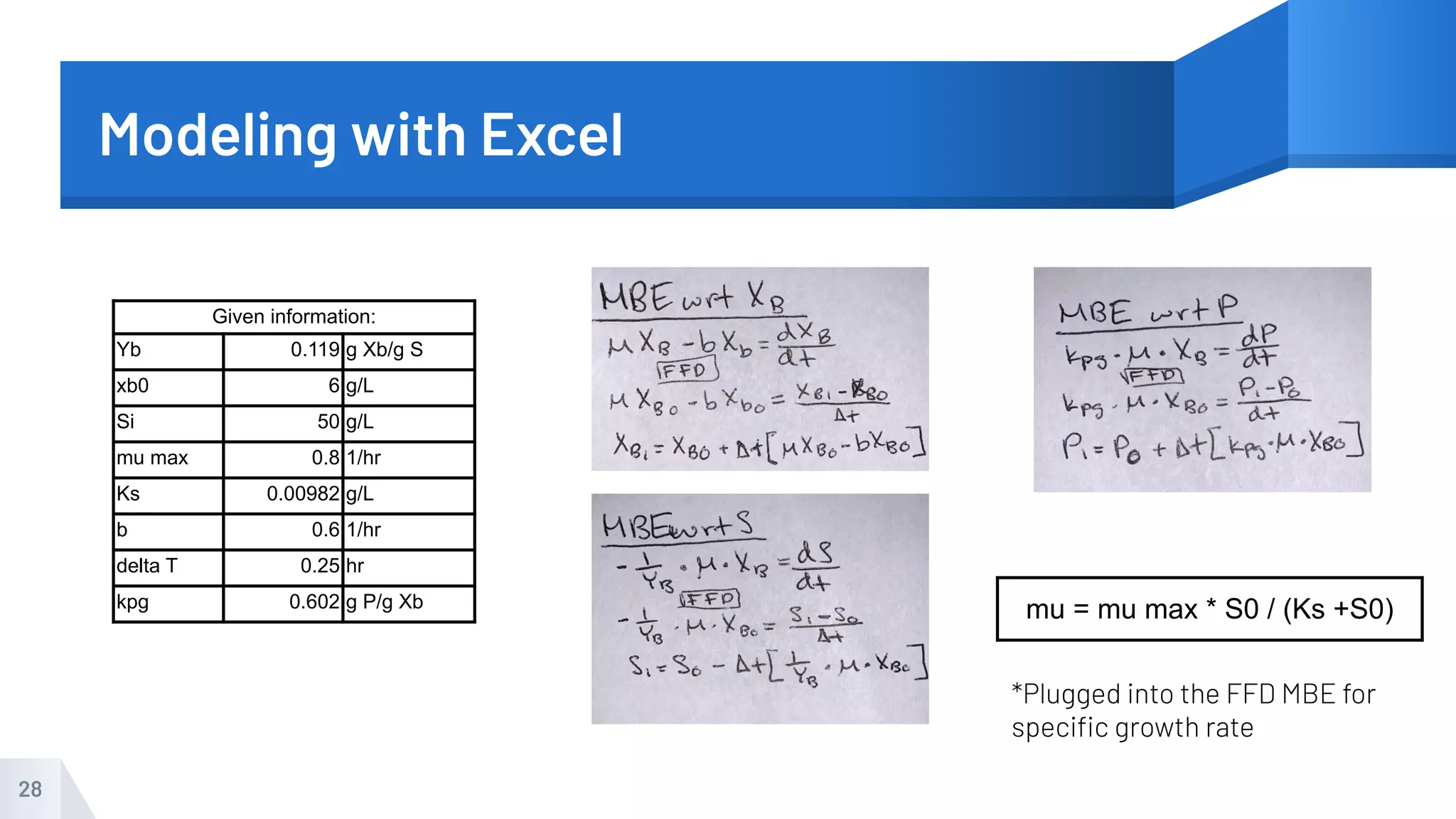
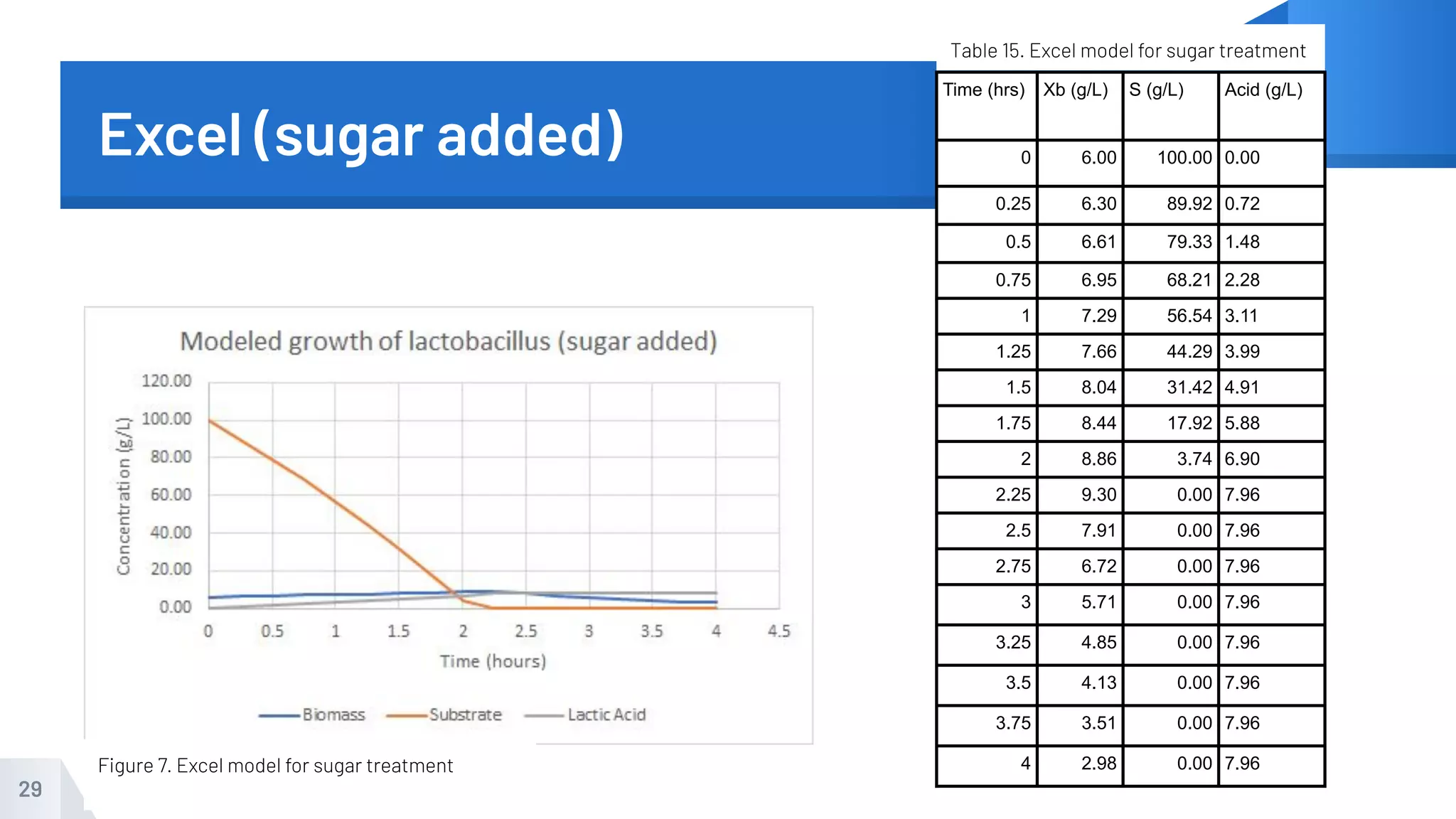
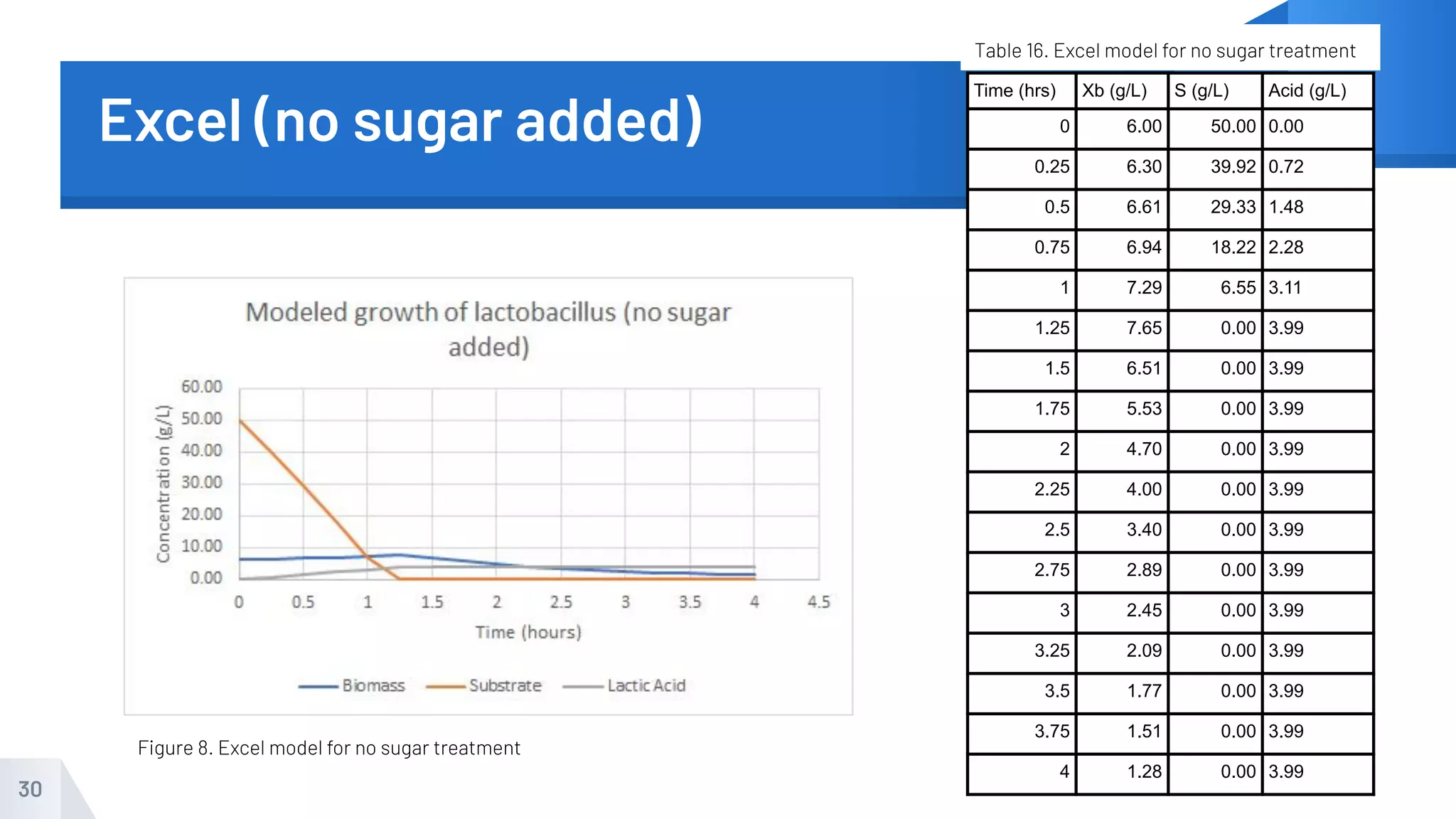
The document describes an experiment to produce yogurt with variable sugar concentrations. Skim milk was fermented with yogurt starter culture both with and without added sugar. Parameters like pH, viscosity, temperature, titration, and COD absorbance were measured over 4 hours to compare fermentation. Modeling of the process was also done in Stella and Excel. Results showed only minor viscosity increases and no clear trend of one scenario fermenting more successfully. Improvements suggested were more precise equipment, controls, and modeling with more appropriate sugar levels.