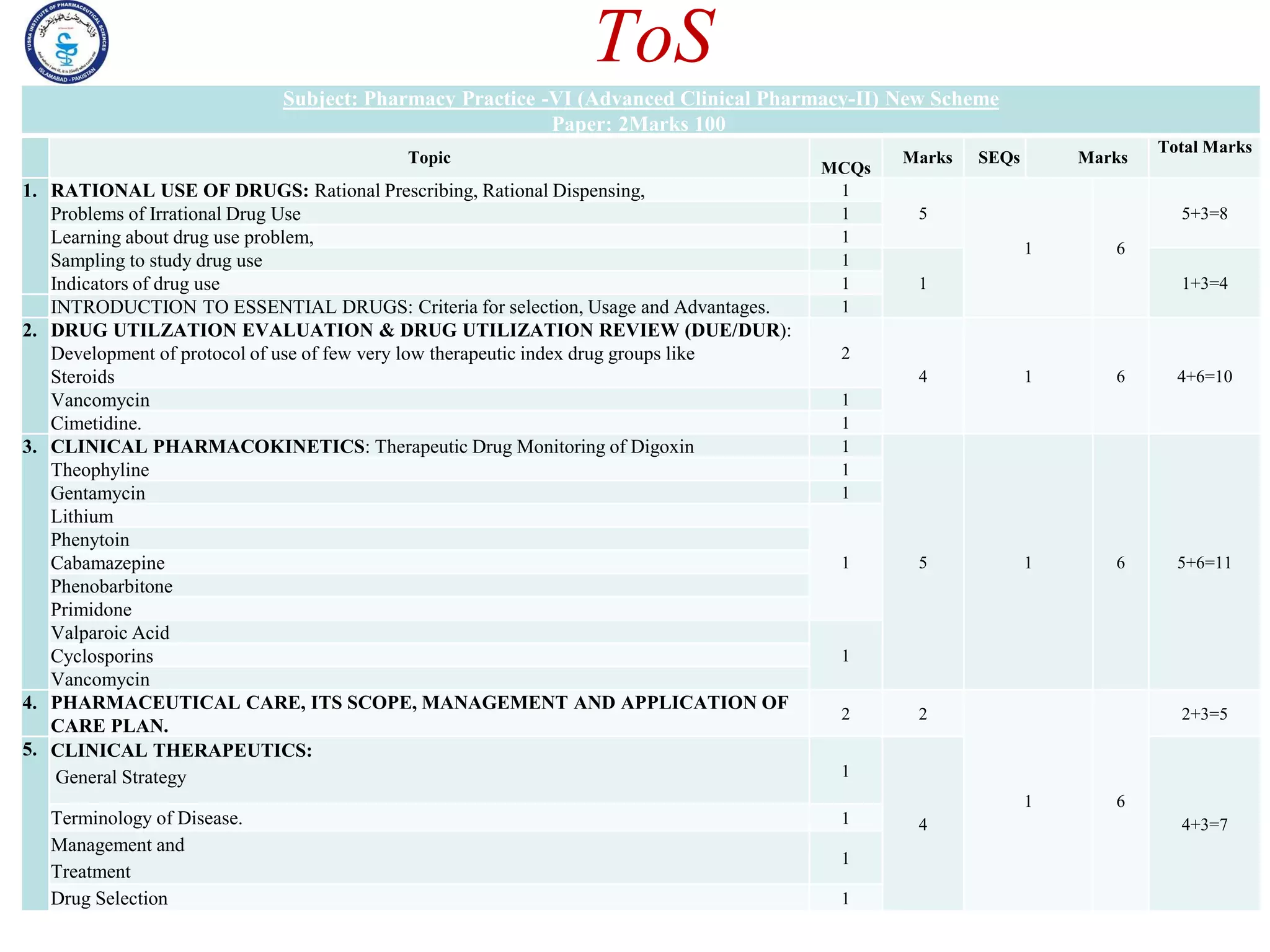
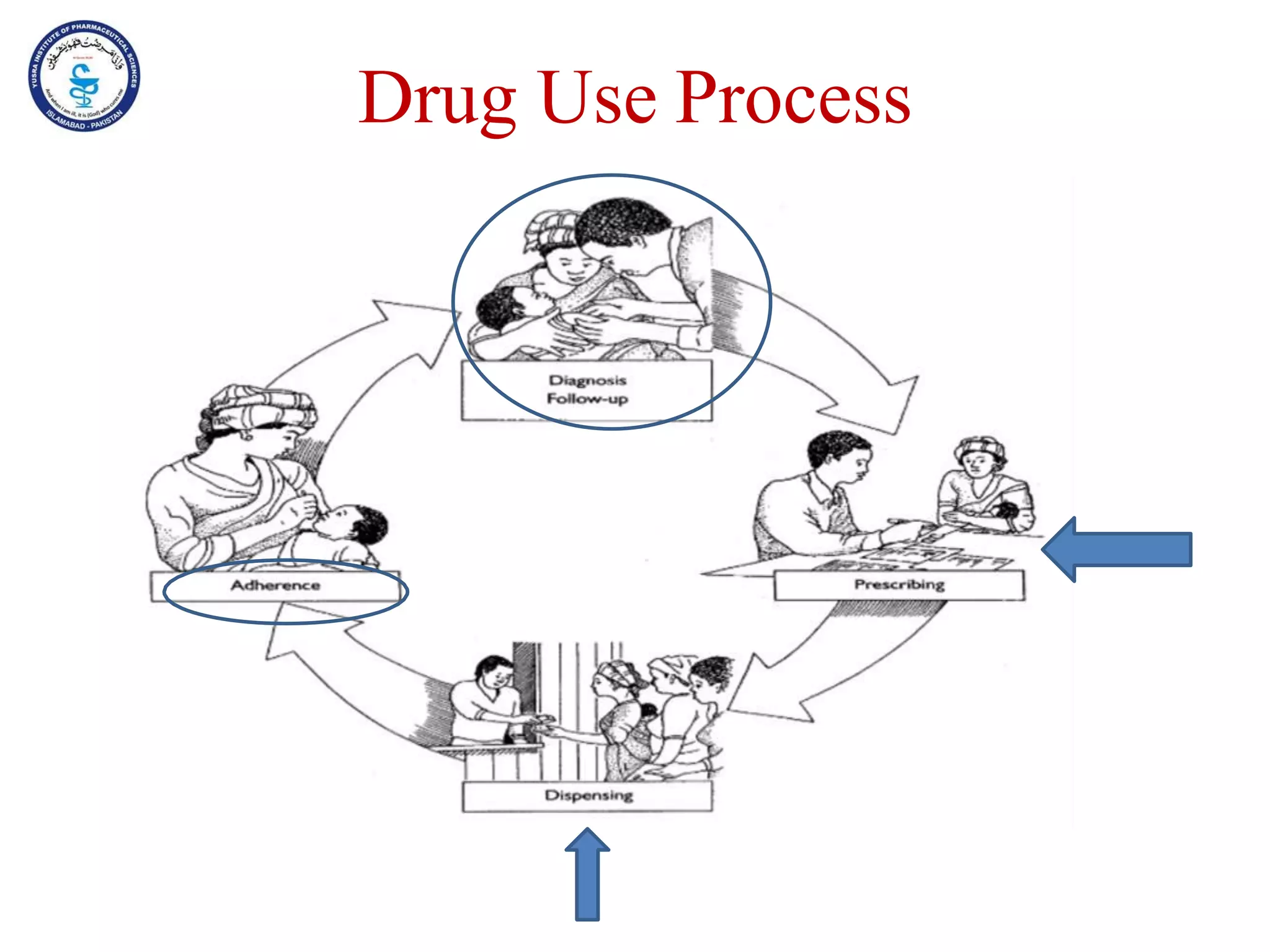
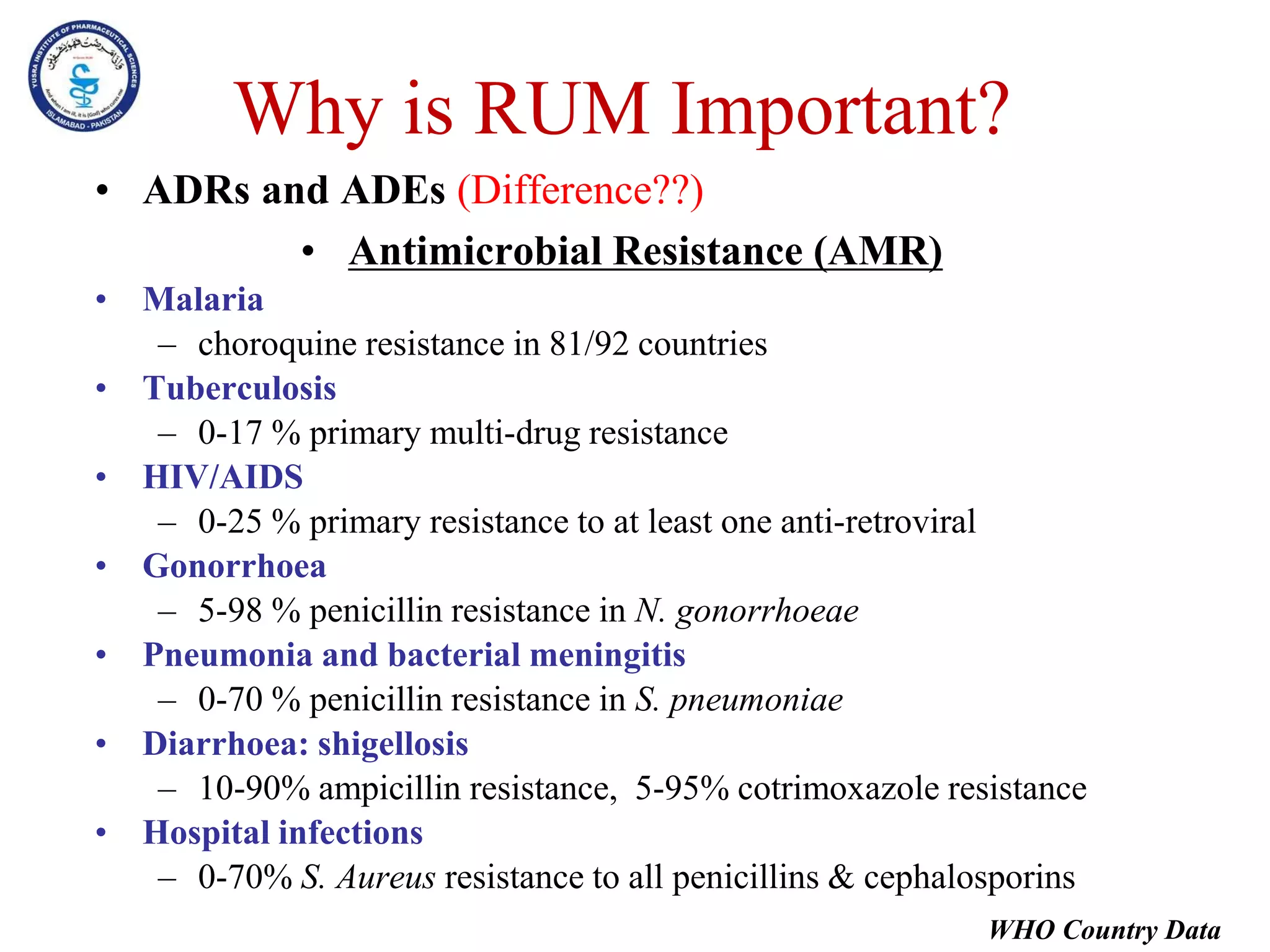
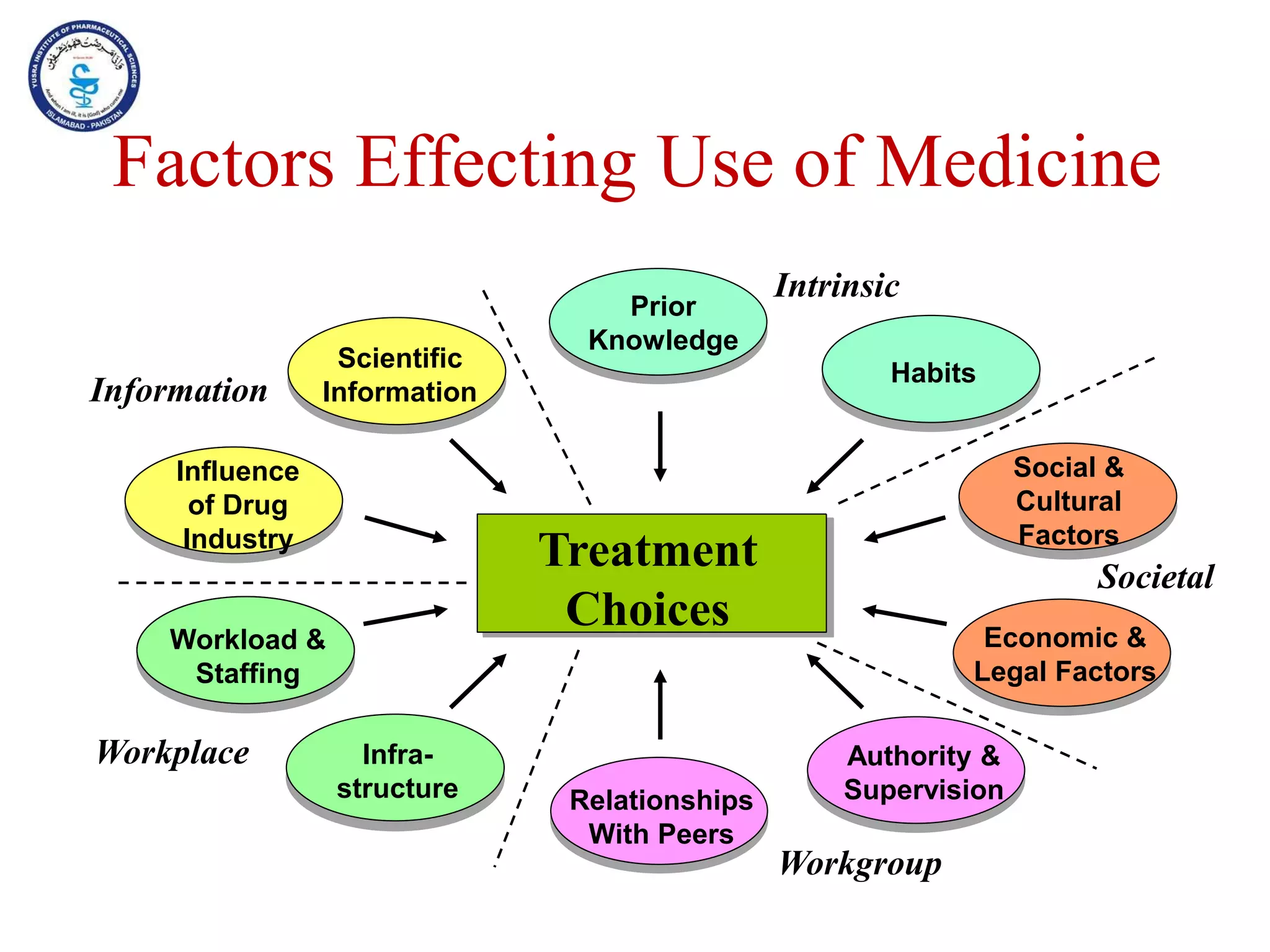
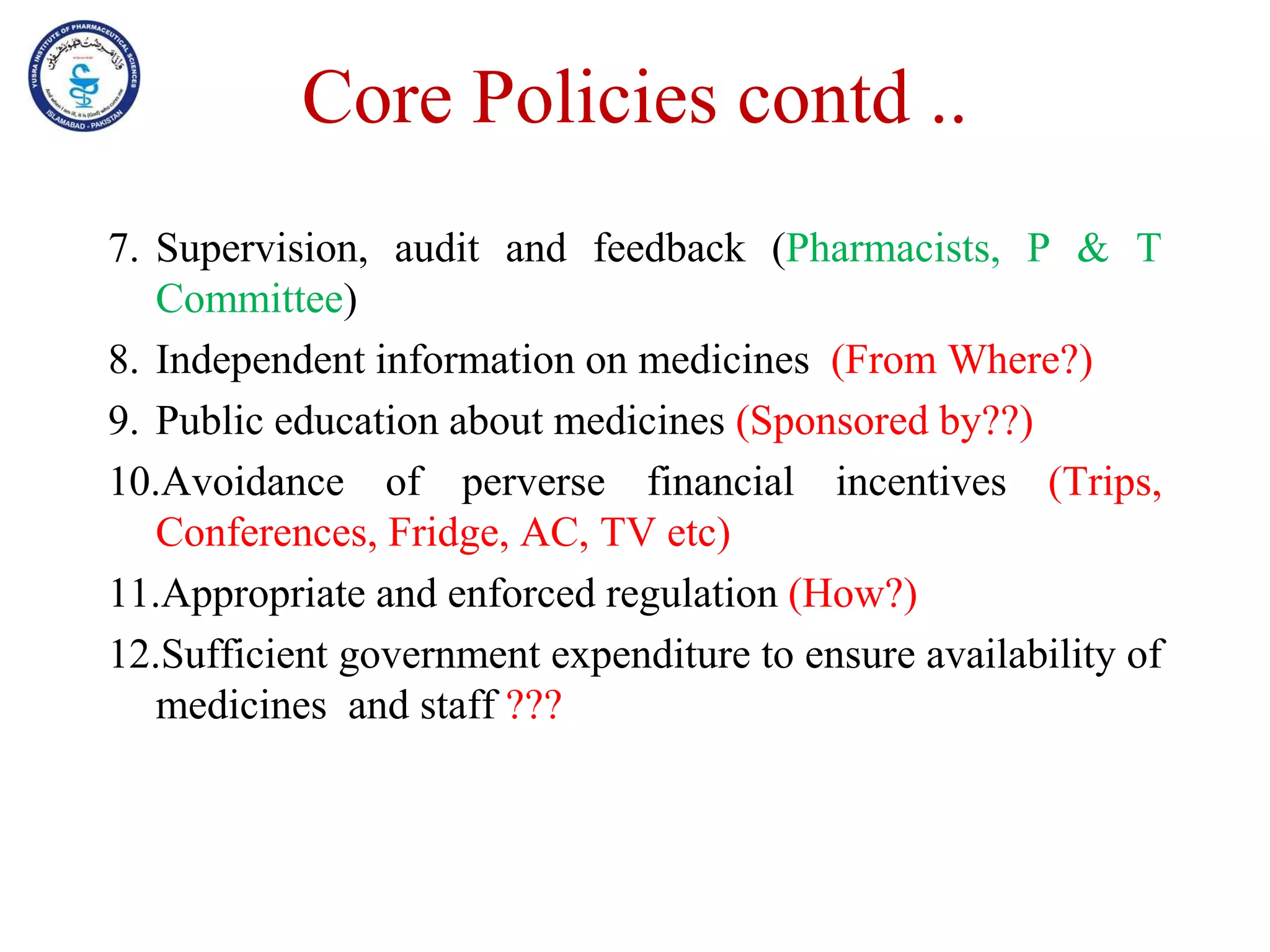
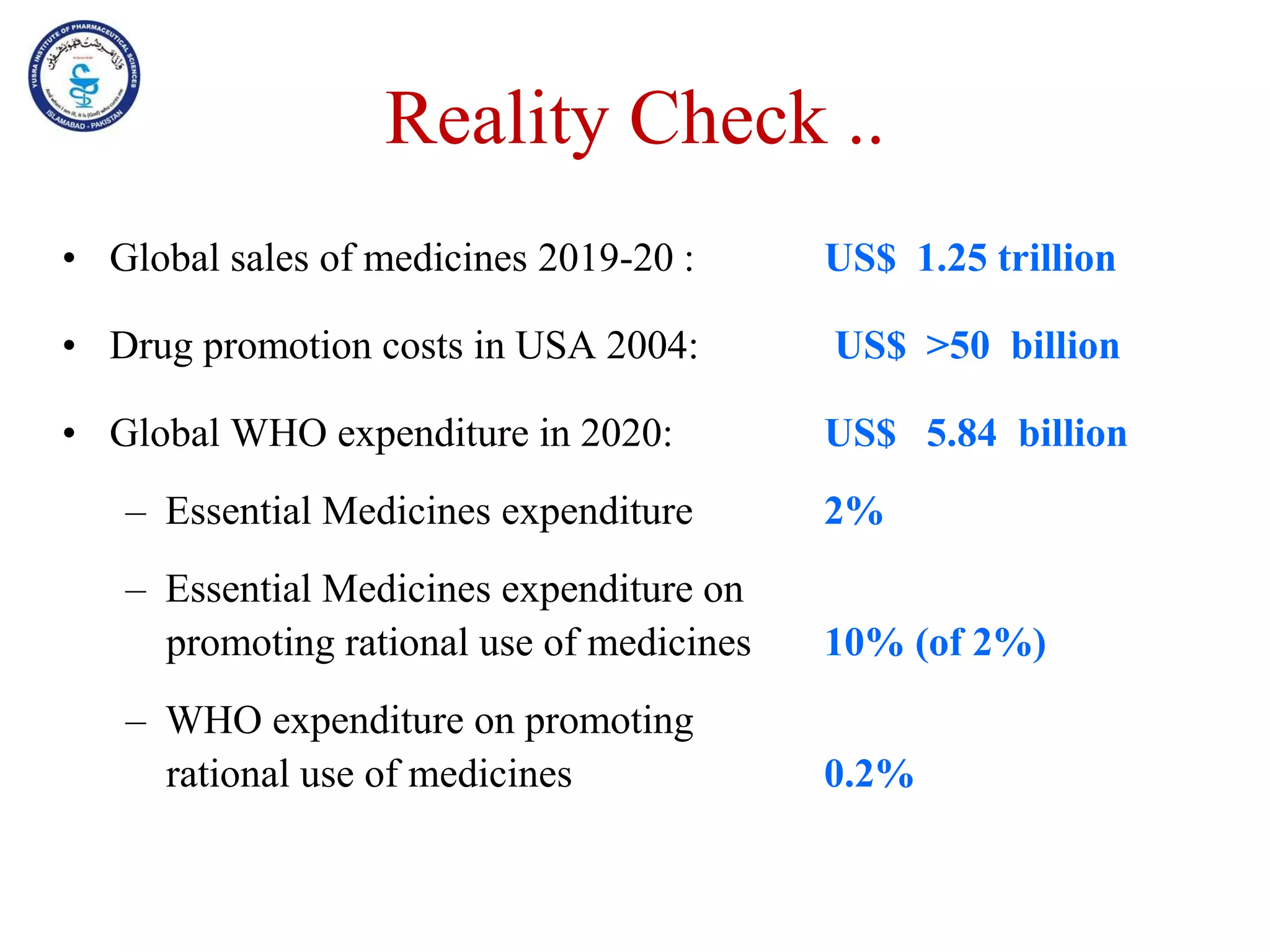
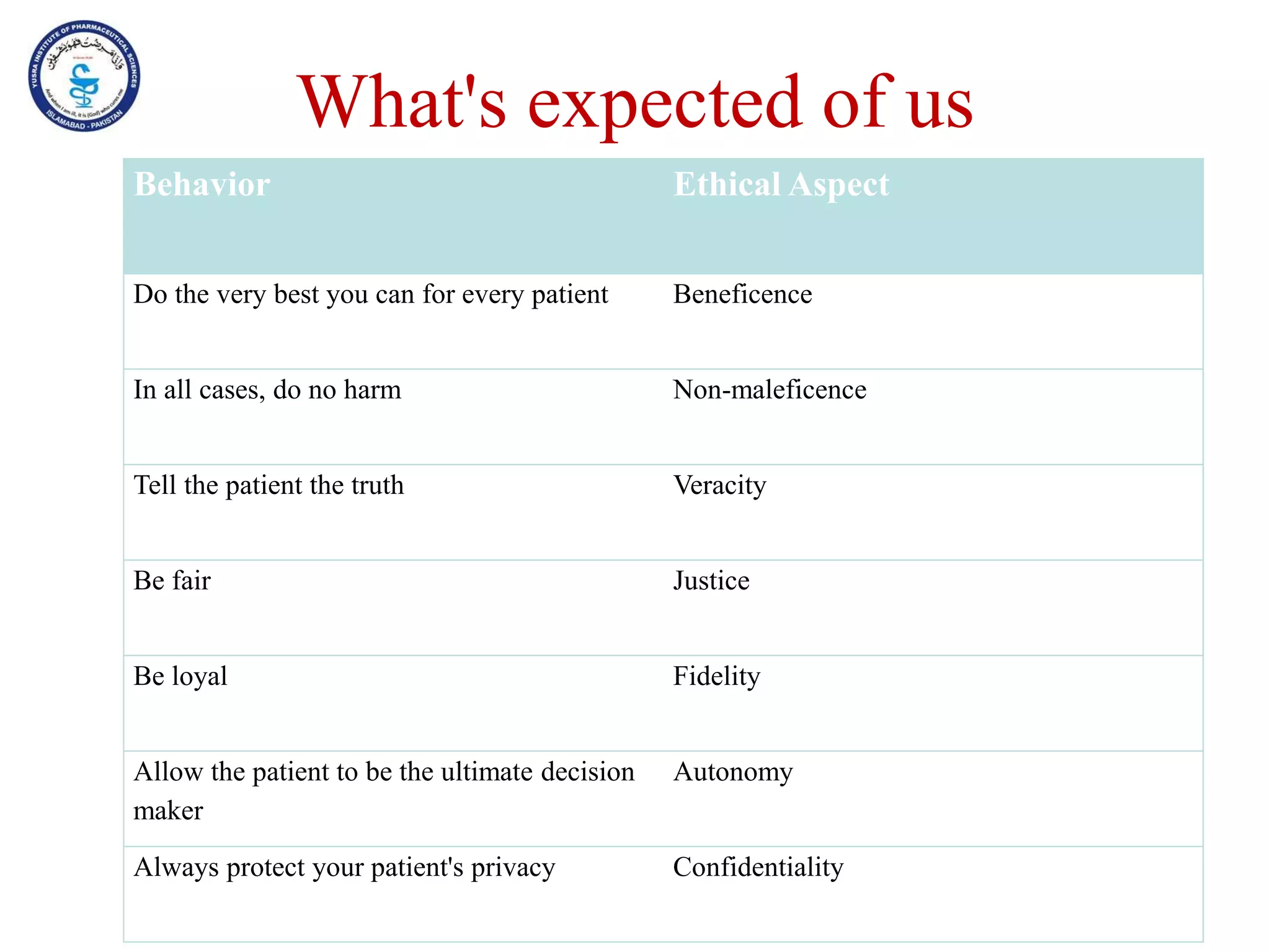
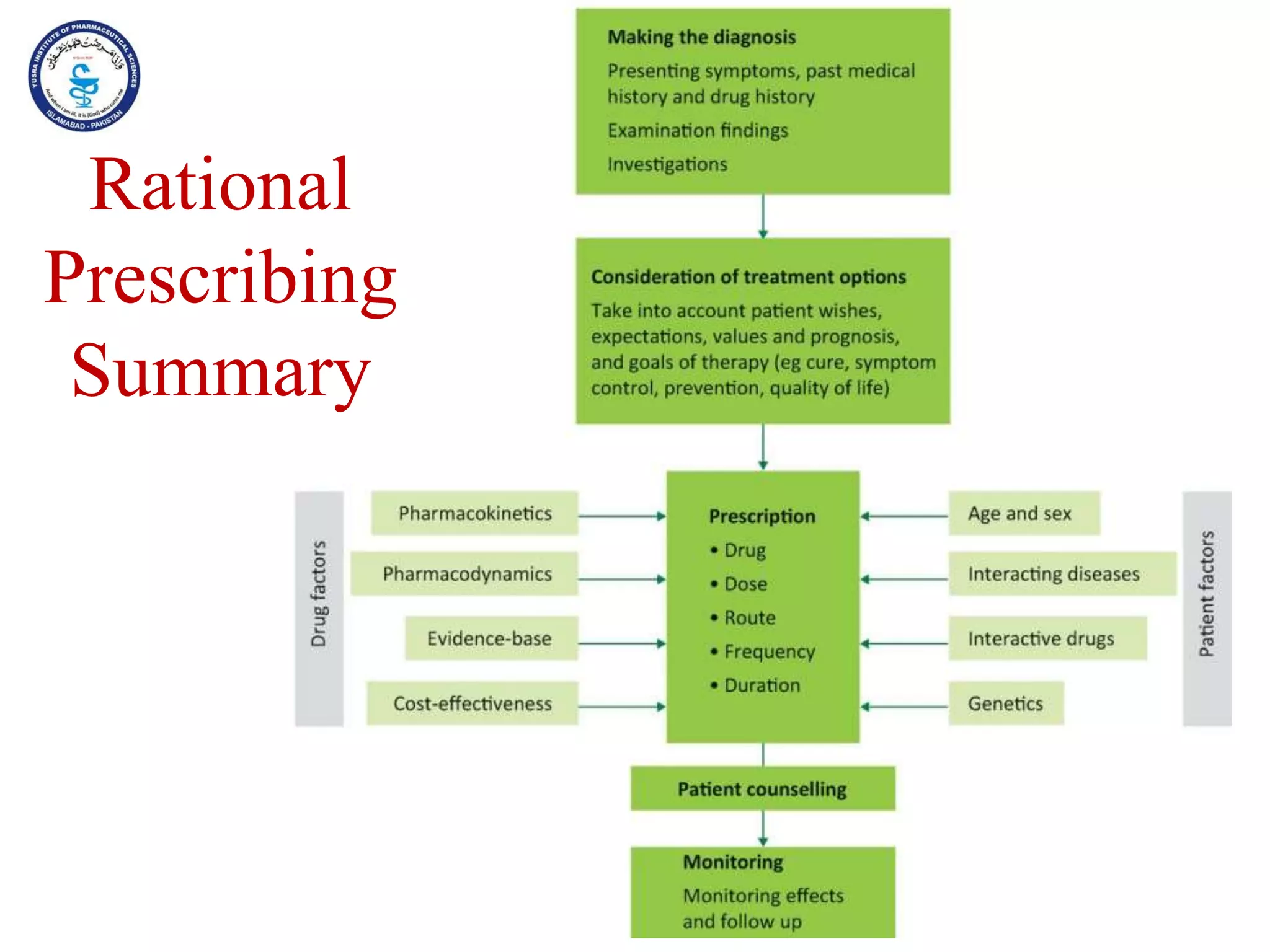
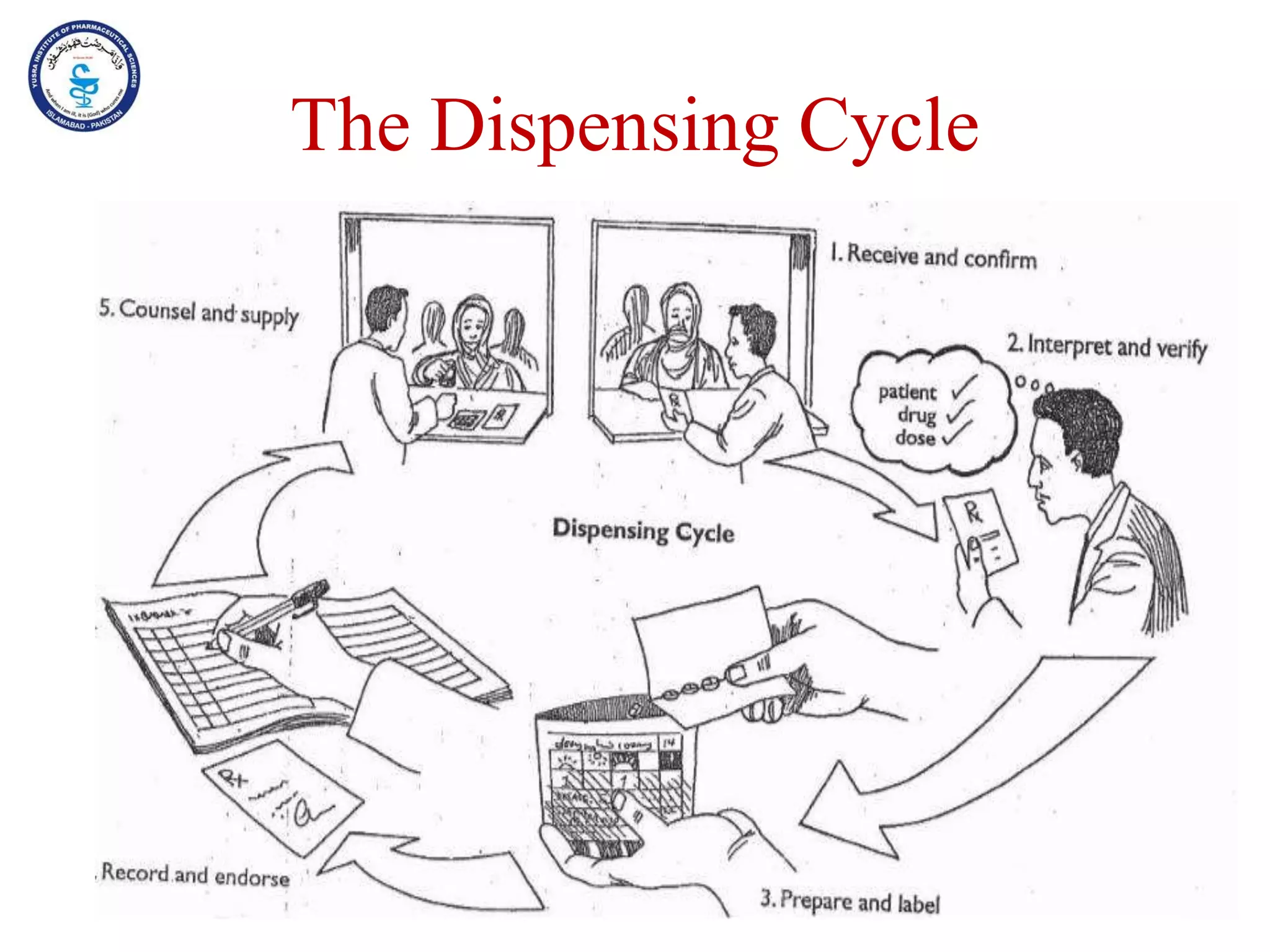
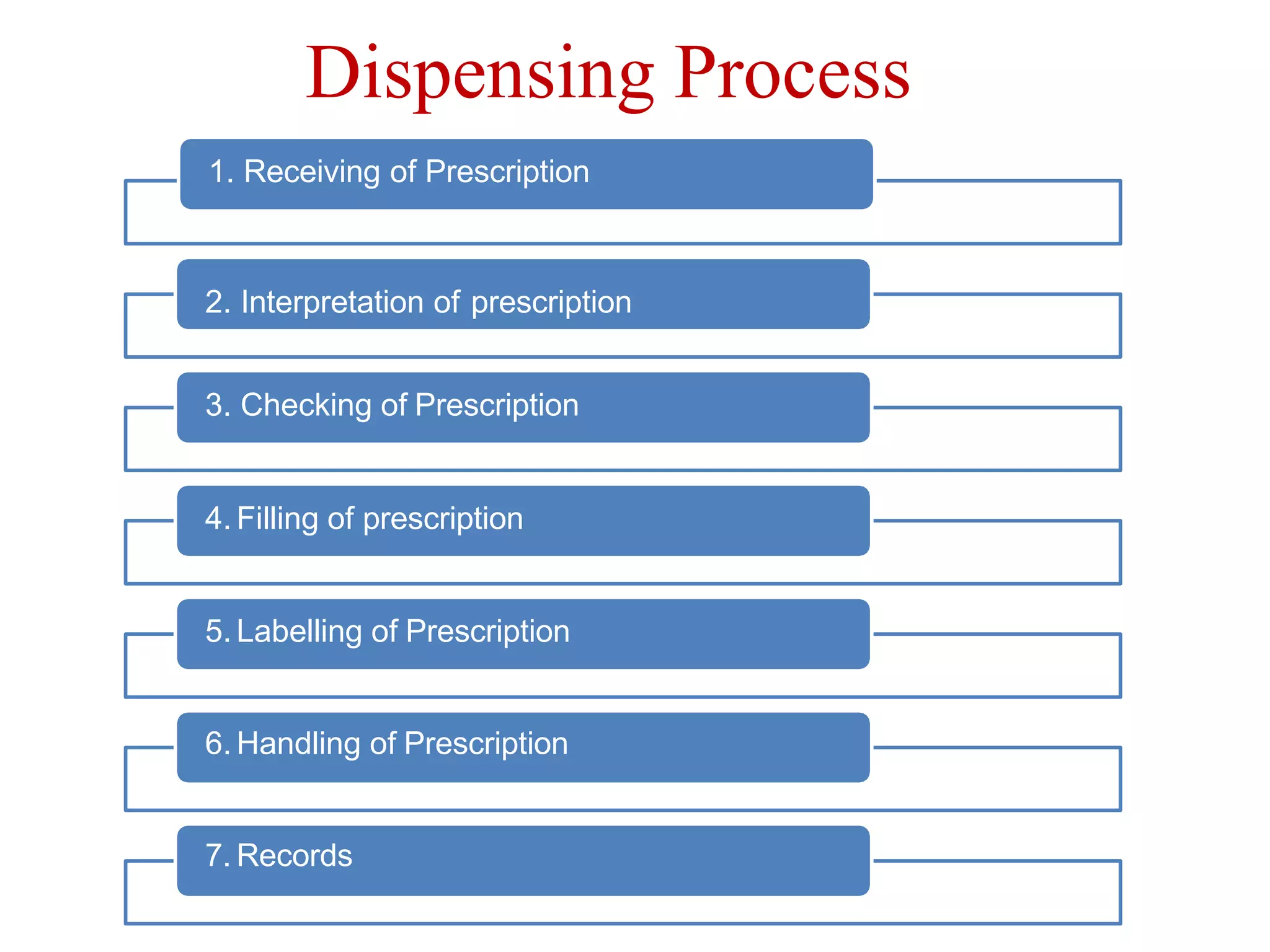
This document outlines the topics that will be covered in the Clinical Pharmacy II course, including rational use of drugs, rational prescribing, rational dispensing, problems of irrational drug use, and factors causing irrational drug use. It provides the course contents, marking scheme, topics to be covered, and expected learning outcomes. The key areas of focus for rational use of drugs are ensuring patients receive appropriate medications to meet their clinical needs at the lowest possible cost. Rational prescribing involves selecting drugs based on safety, effectiveness, tolerability, price and simplicity, while rational dispensing aims to provide patients with adequate counseling and instructions for proper medication use. Irrational drug use can result from improper prescribing, dispensing, or non-adherence and lead to negative