This document summarizes key concepts in thermodynamics taught by Dr. Vishal Patil including:
1. Macroscopic thermodynamics looks at bulk effects of many molecules, while microscopic looks at individual molecules.
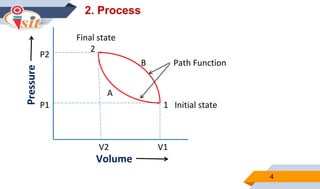
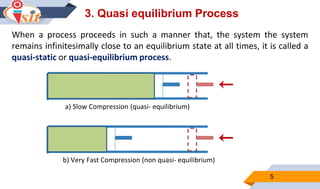
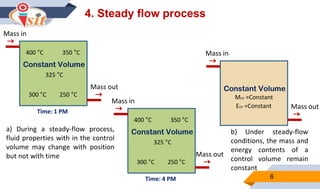
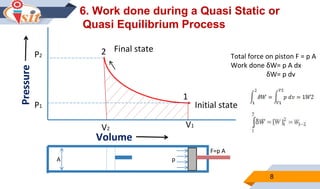
2. Quasi-static processes keep a system infinitesimally close to equilibrium, while steady flow processes have properties change within a control volume but not over time.
3. The zeroth law of thermodynamics states that two bodies in thermal equilibrium with a third are in equilibrium with each other.