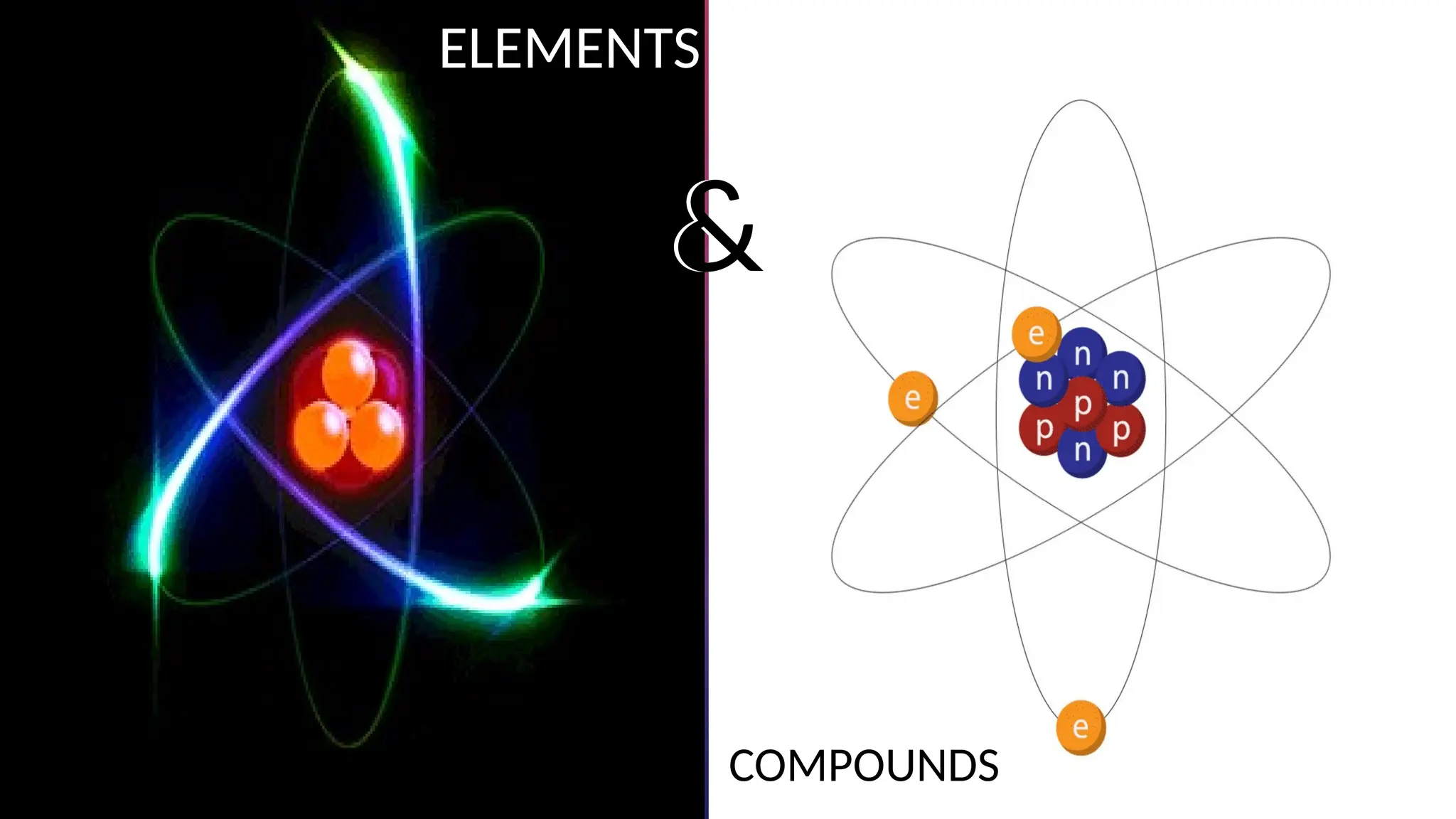
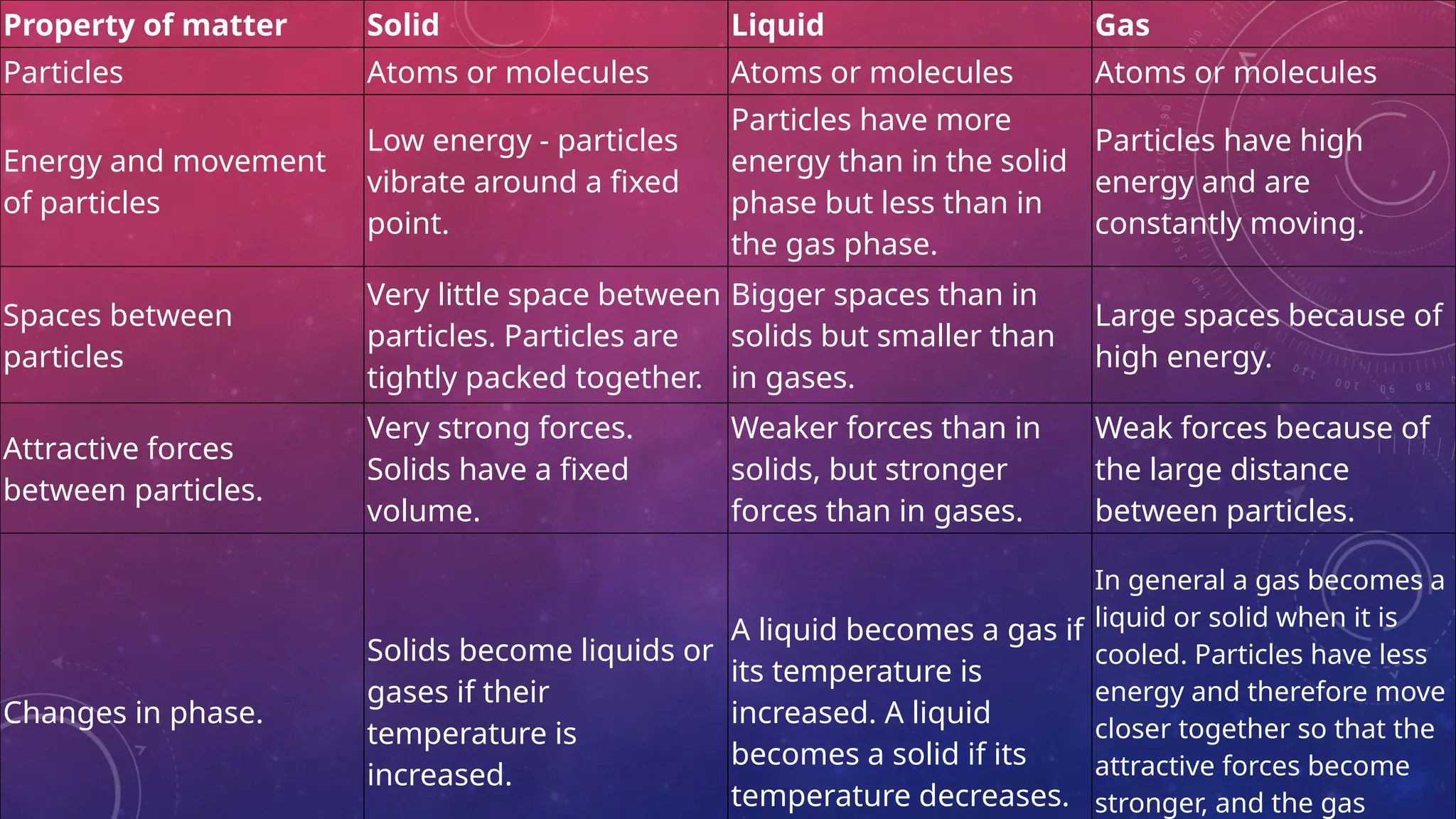
The document consists of questions and answers related to scientific models, the composition of matter, and the kinetic molecular theory. It discusses the purpose of models in science, the classification of pure substances into elements and compounds, and how particle motion relates to different phases of matter. Additionally, there are checkpoints and activities to reinforce vocabulary and understanding of these concepts.