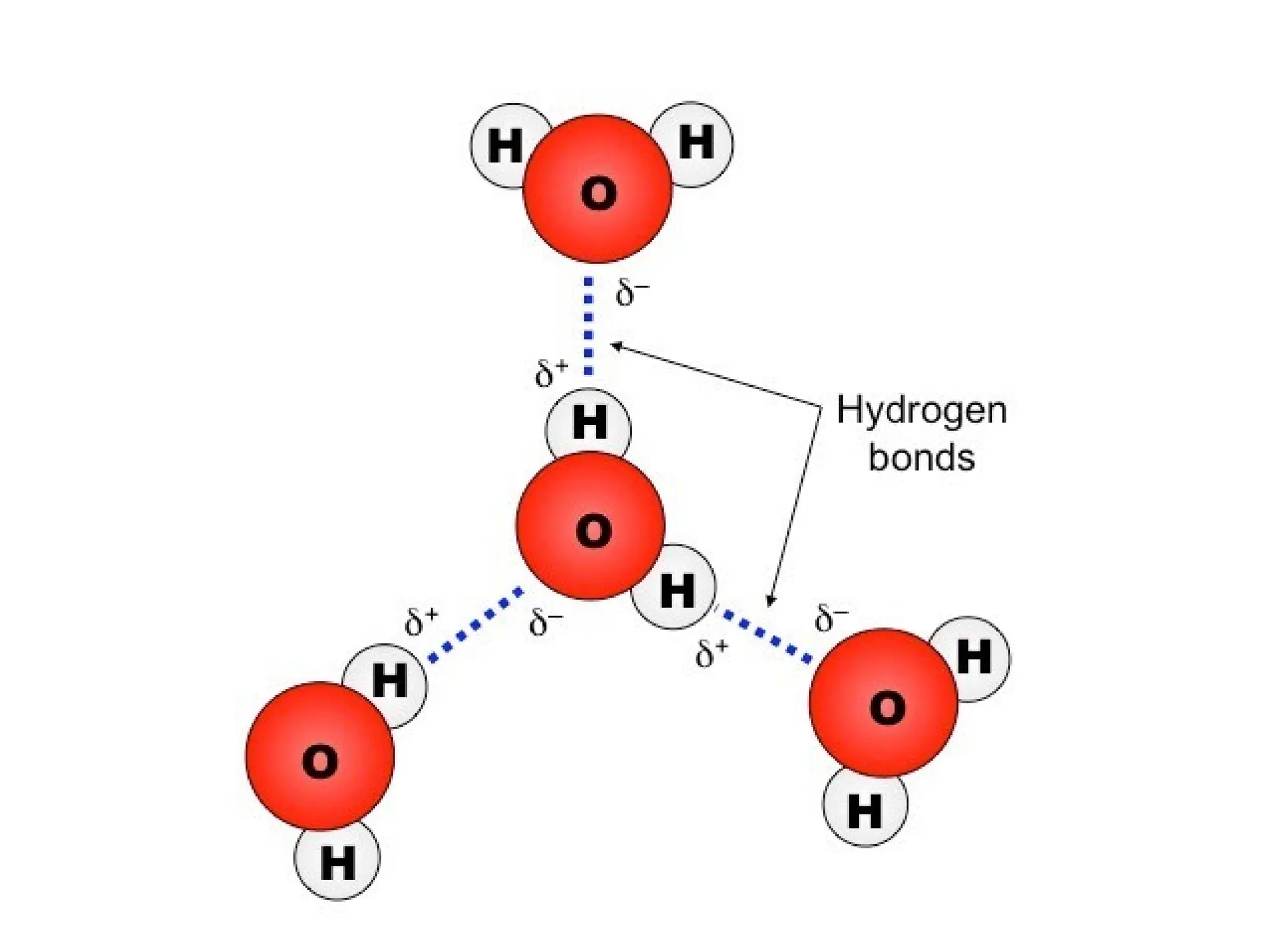
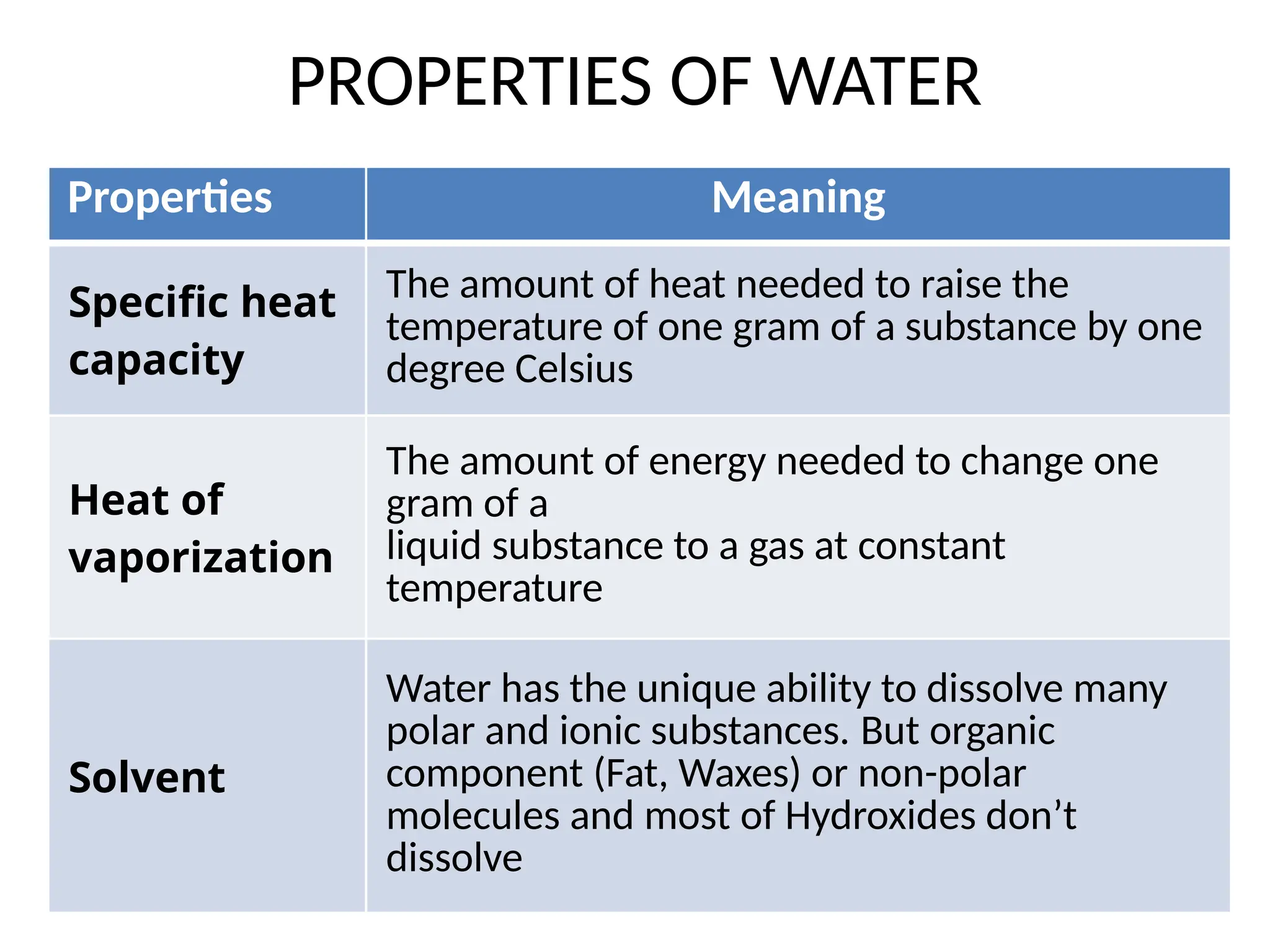
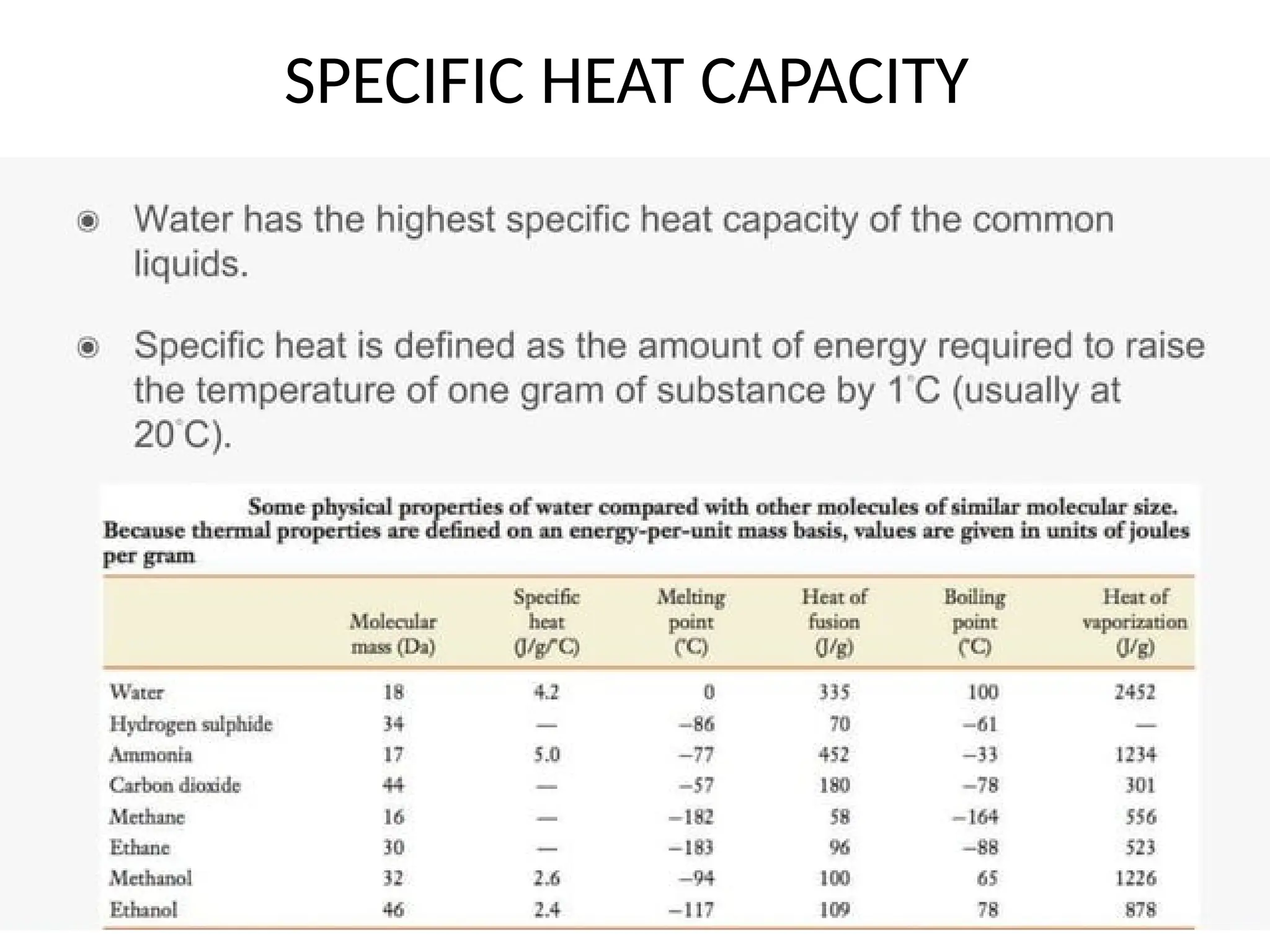
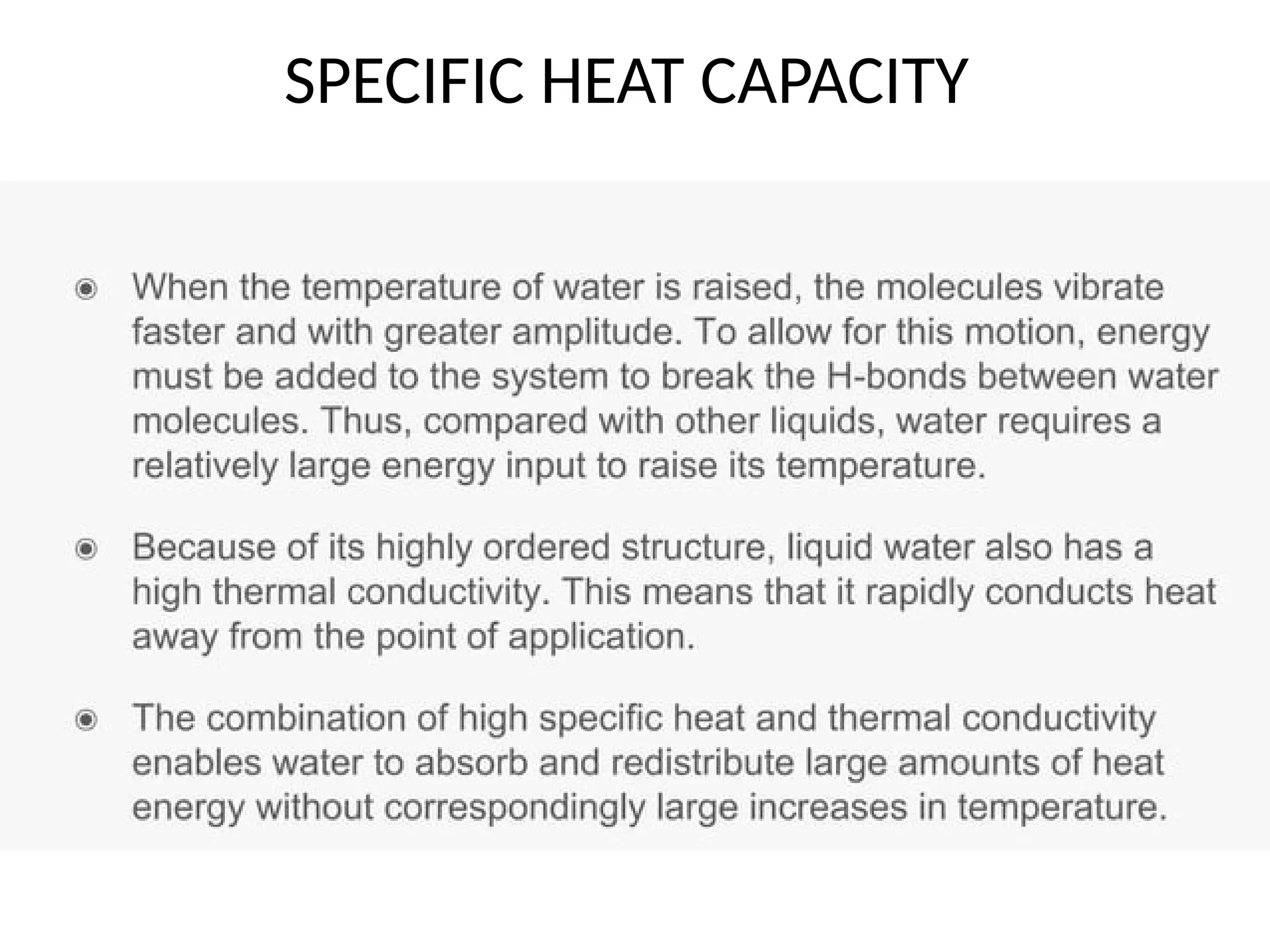
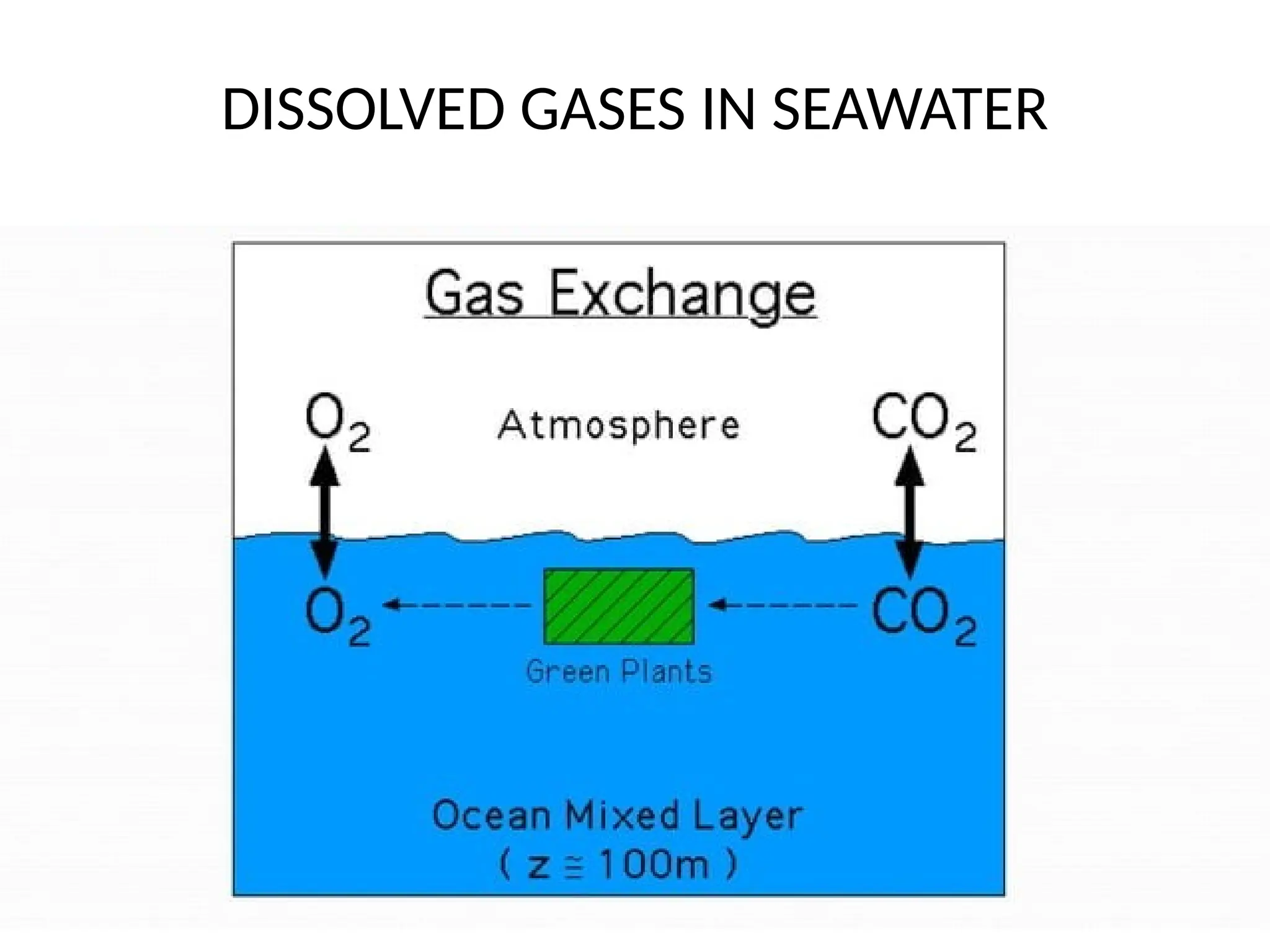
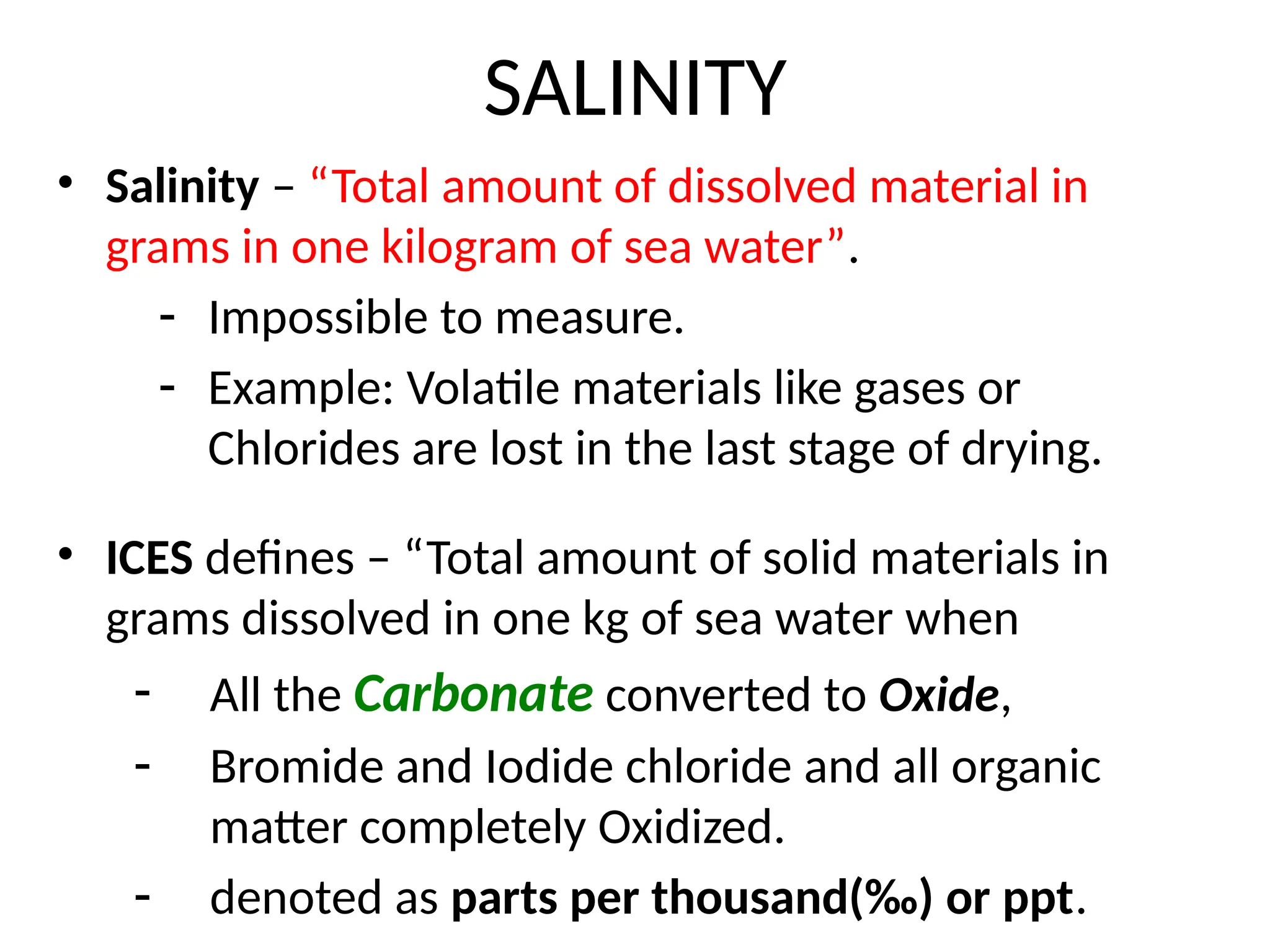
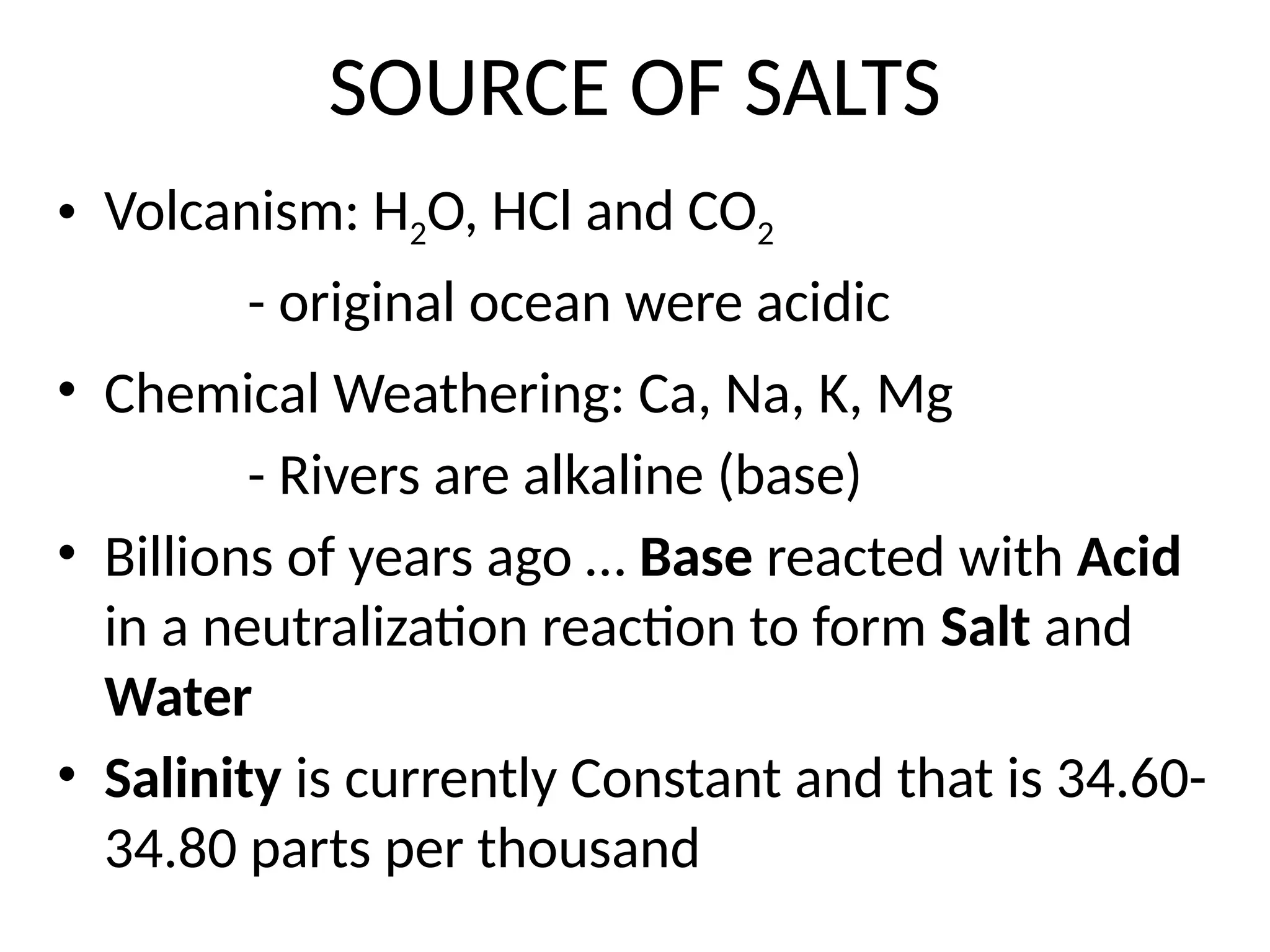
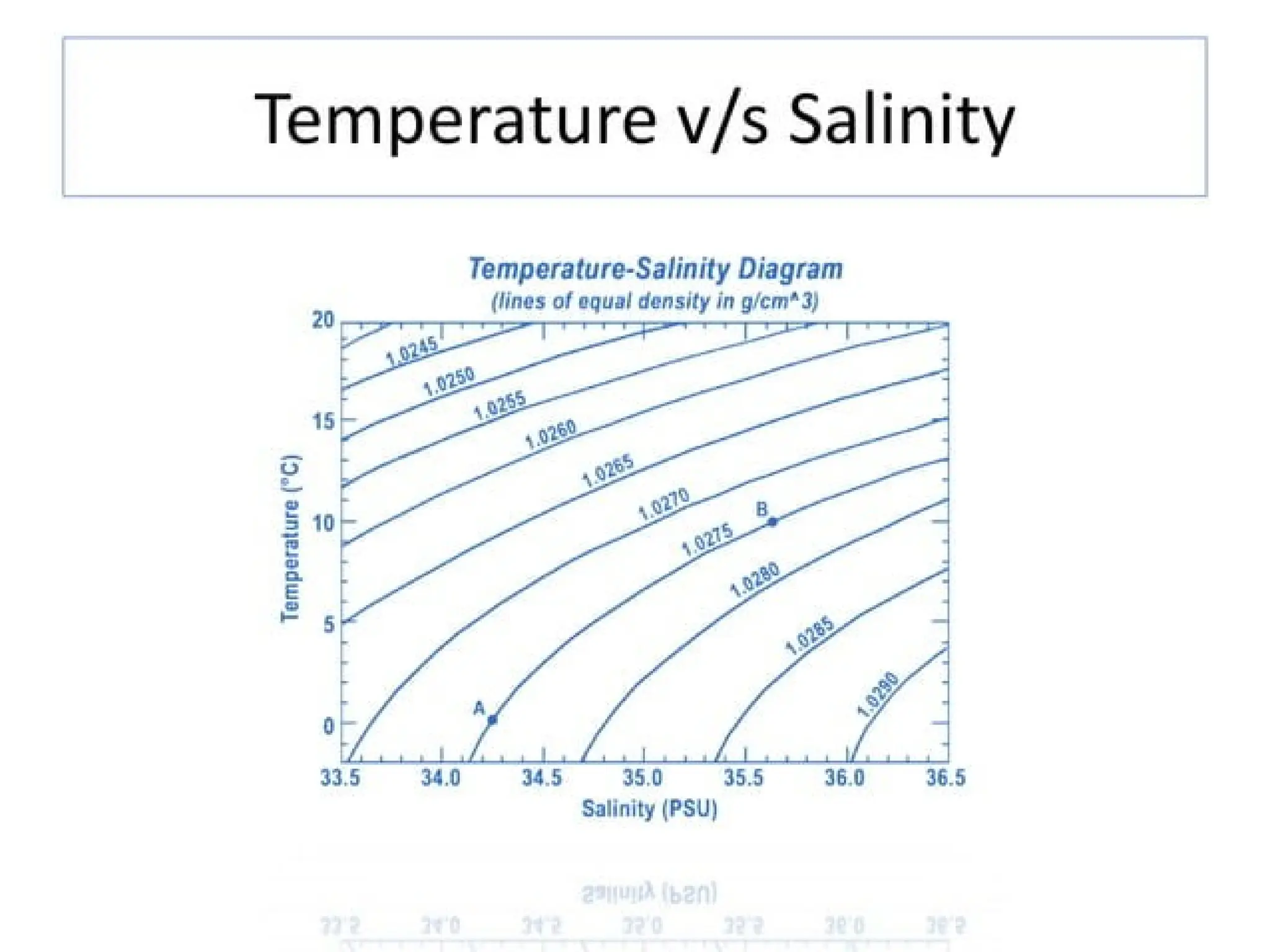
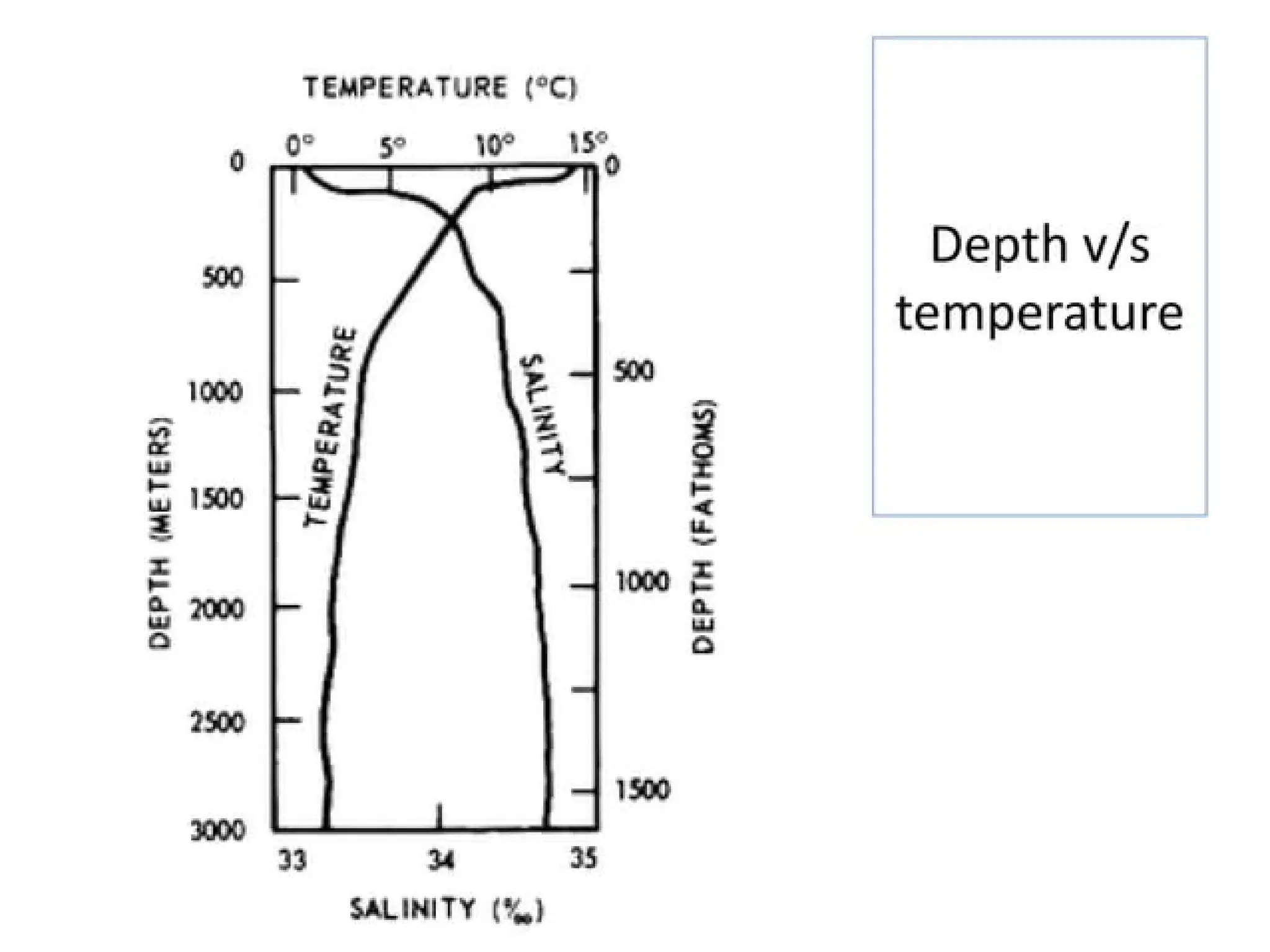
Seawater is the water found in oceans and seas, covering more than 70% of Earth’s surface. It is a complex solution made up of approximately 96.5% water, 2.5% dissolved salts, and smaller amounts of other substances, including inorganic and organic materials, particulates, and trace atmospheric gases.
Seawater is a valuable source of various commercially important elements. A significant portion of the world’s magnesium is extracted from it, along with large amounts of bromine. In some regions, sodium chloride (table salt) is still produced by evaporating seawater. Additionally, desalinated seawater offers an unlimited source of drinking water. To address freshwater shortages, especially in arid coastal regions like the Middle East, many large desalination plants have been established.