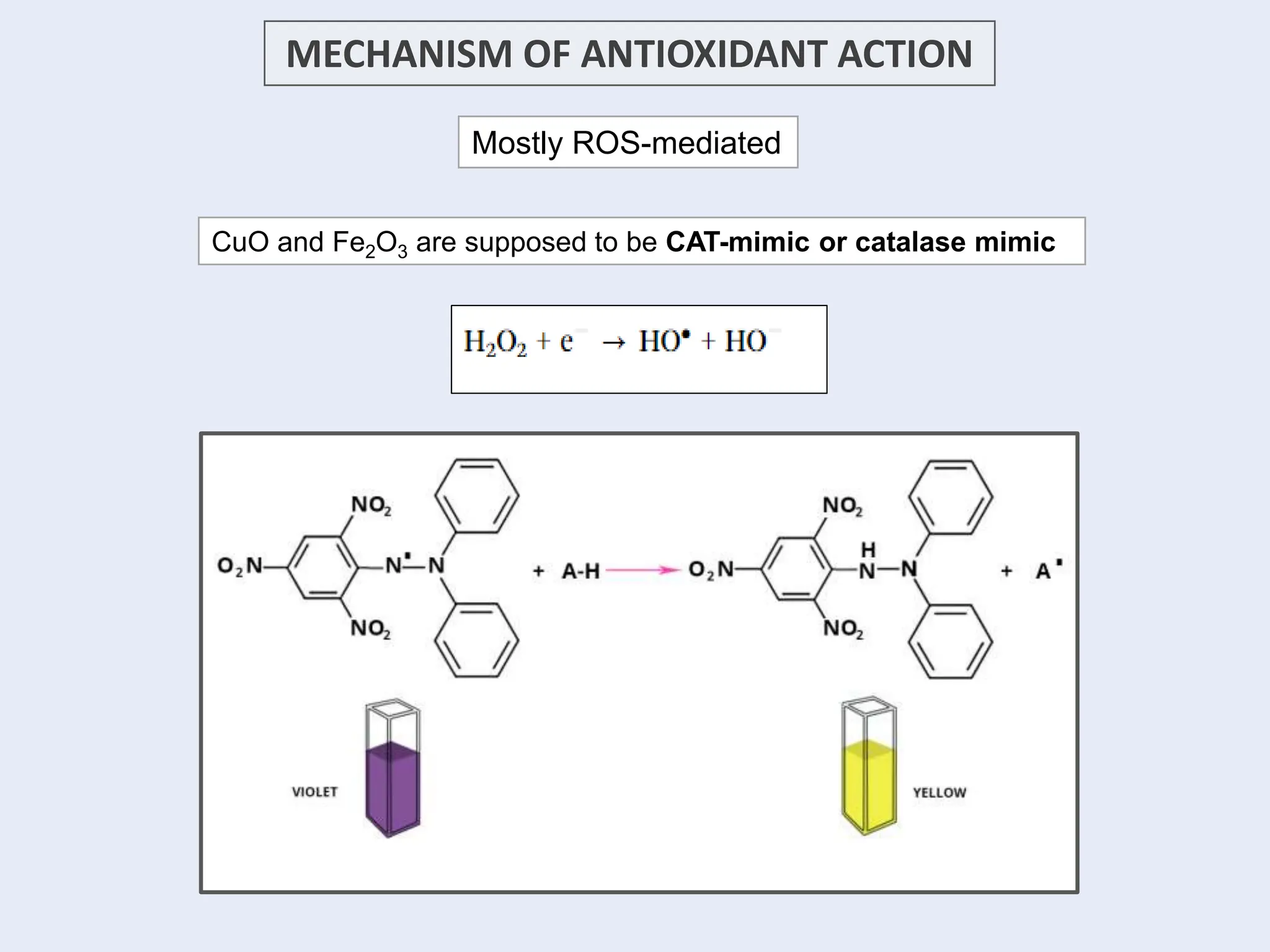
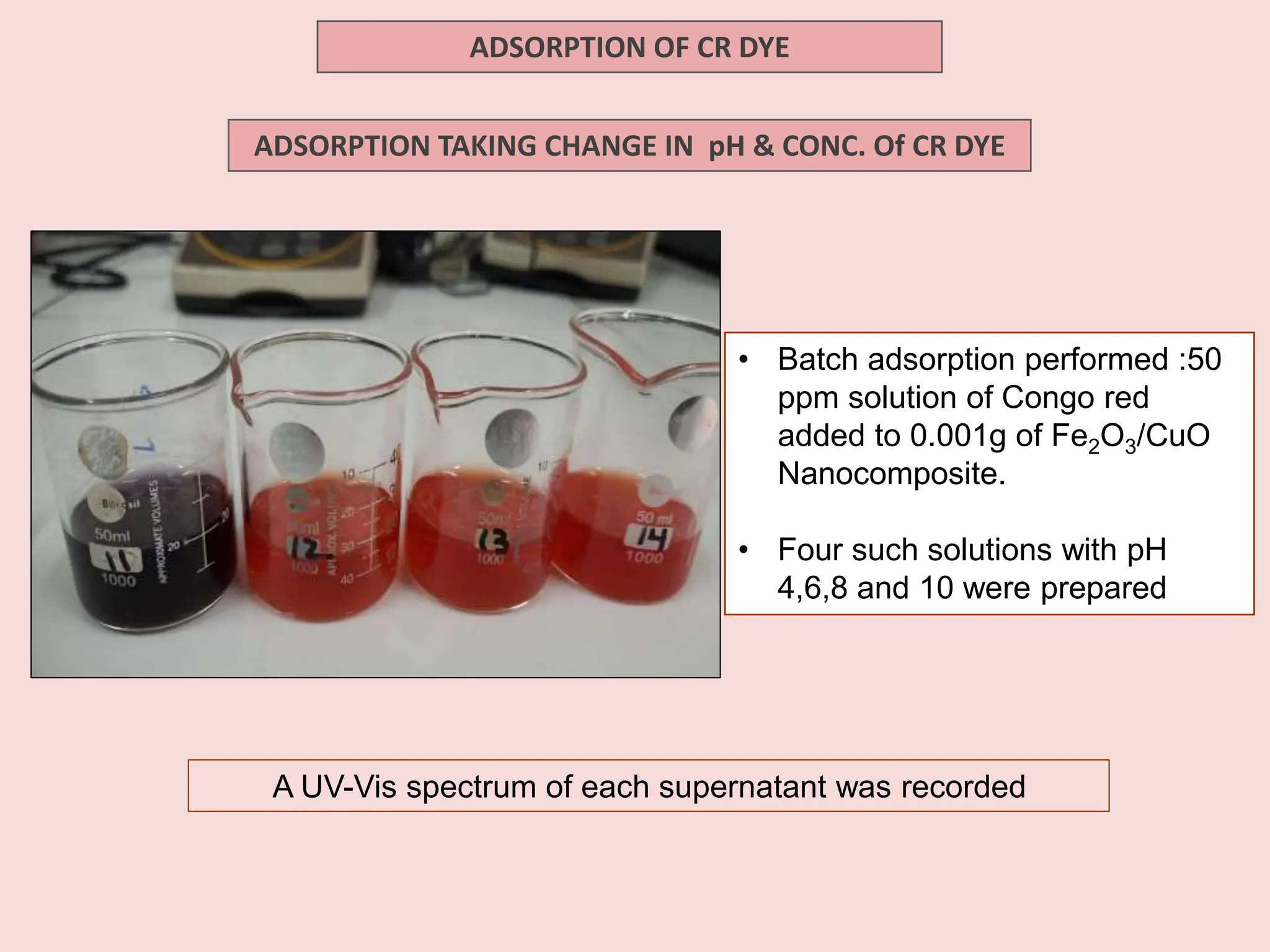
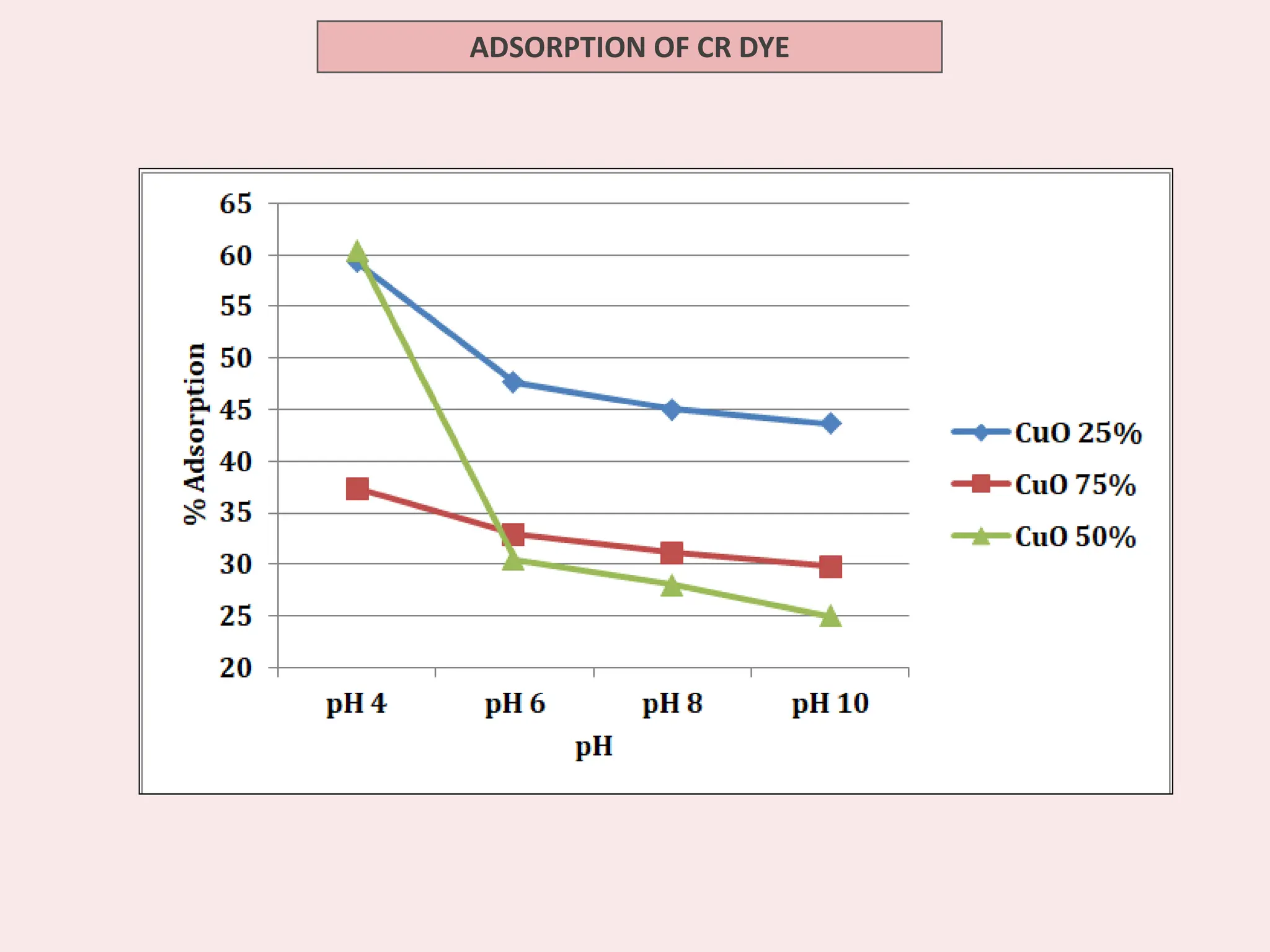
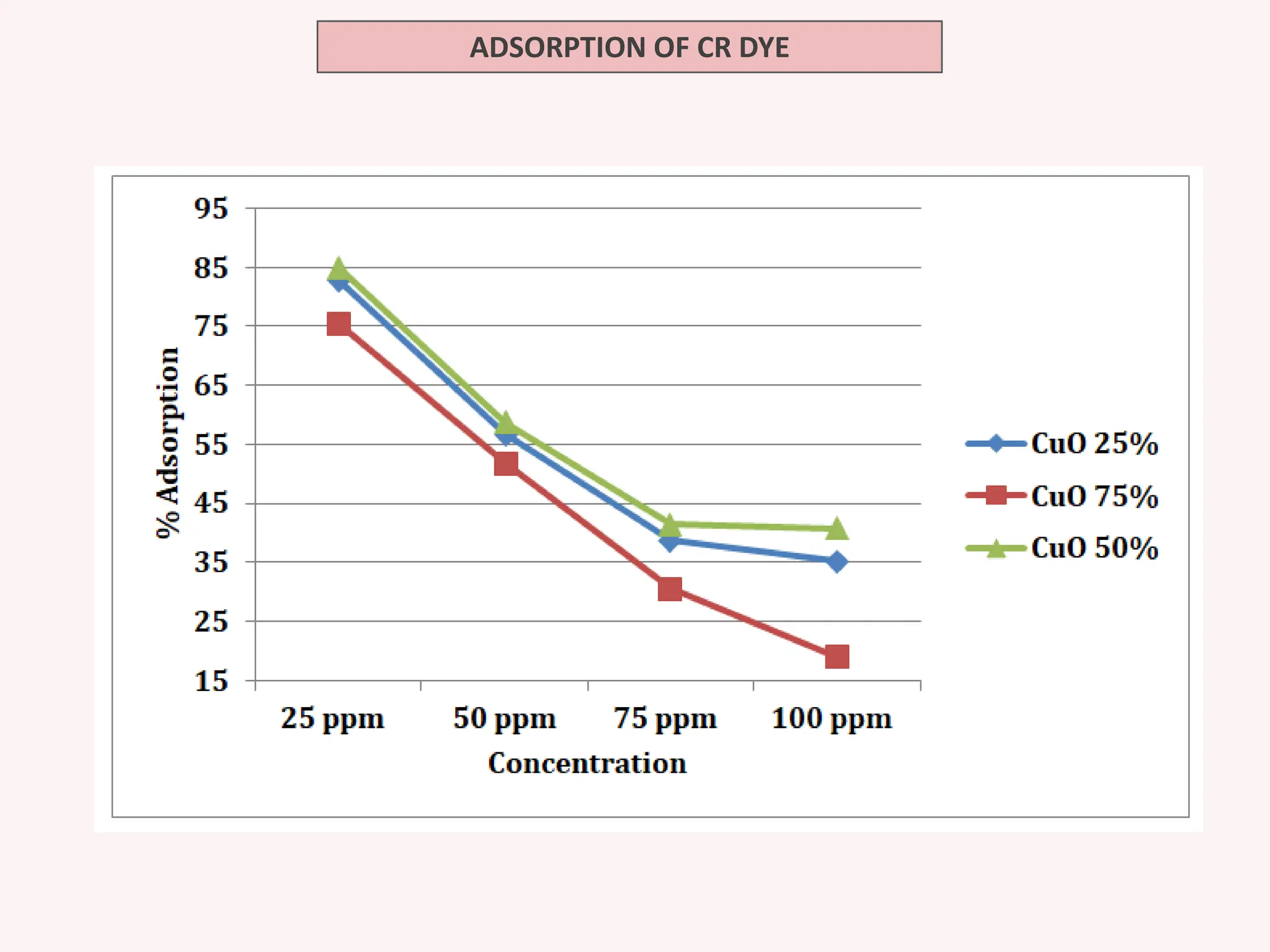
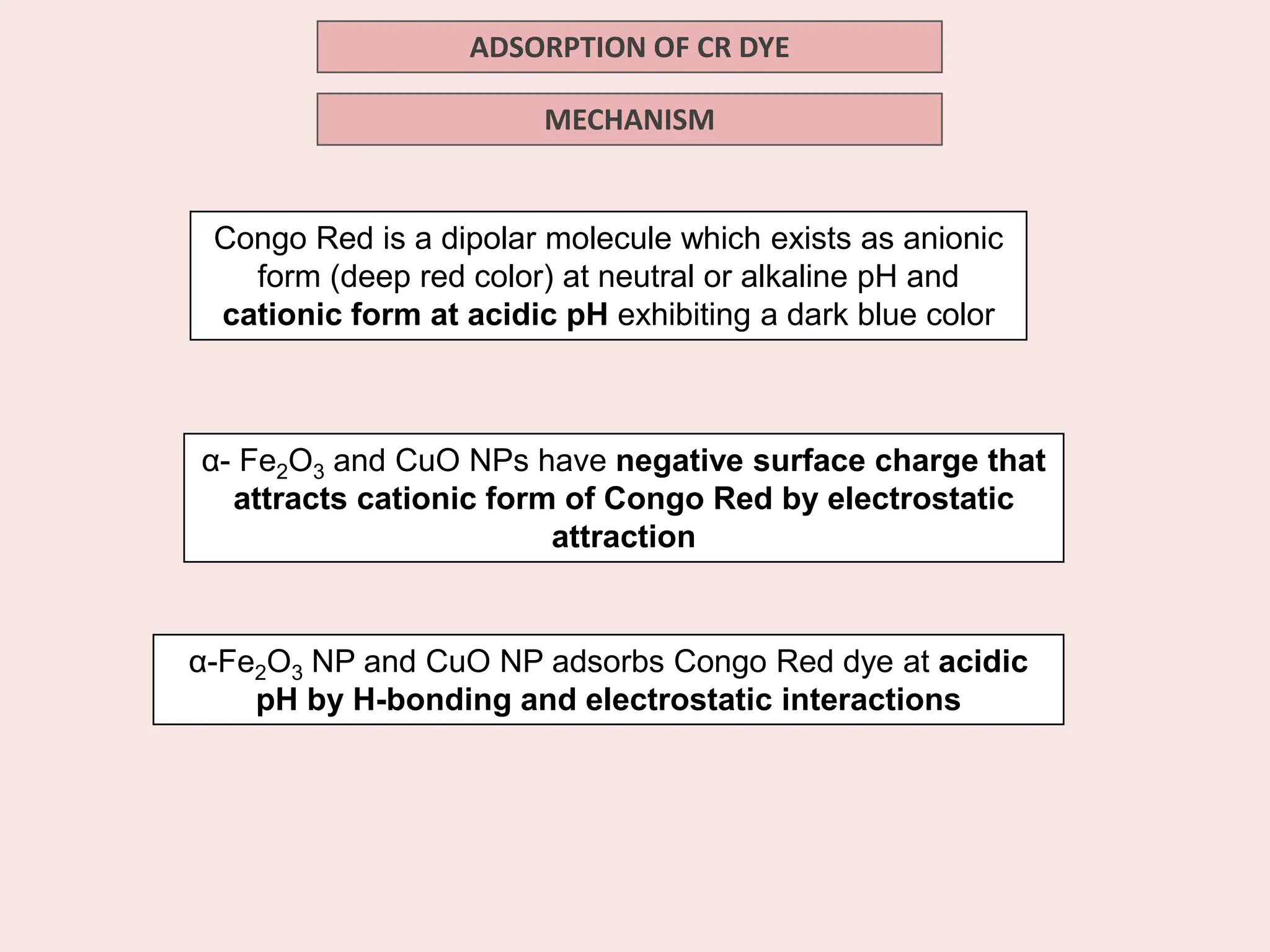
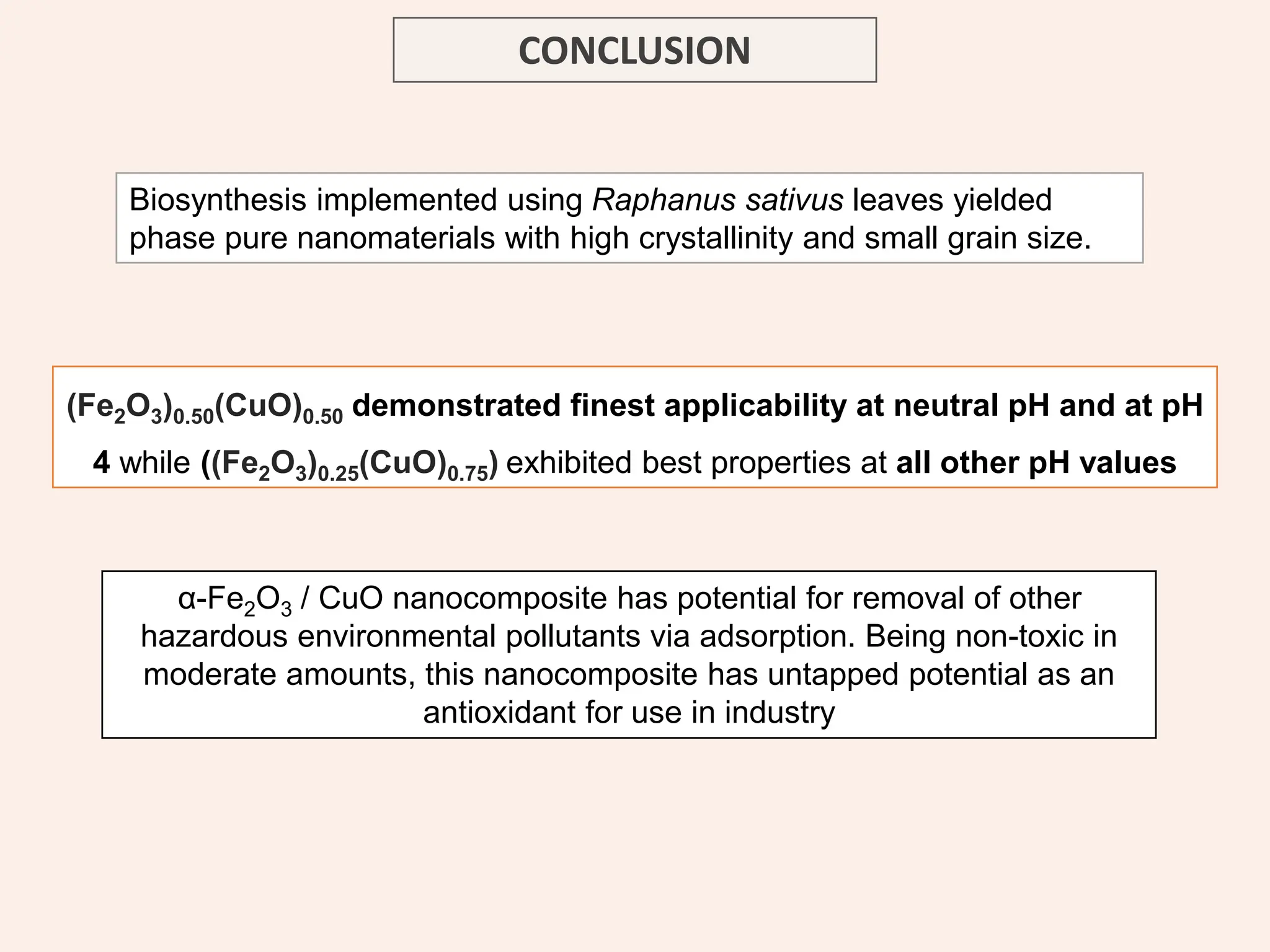
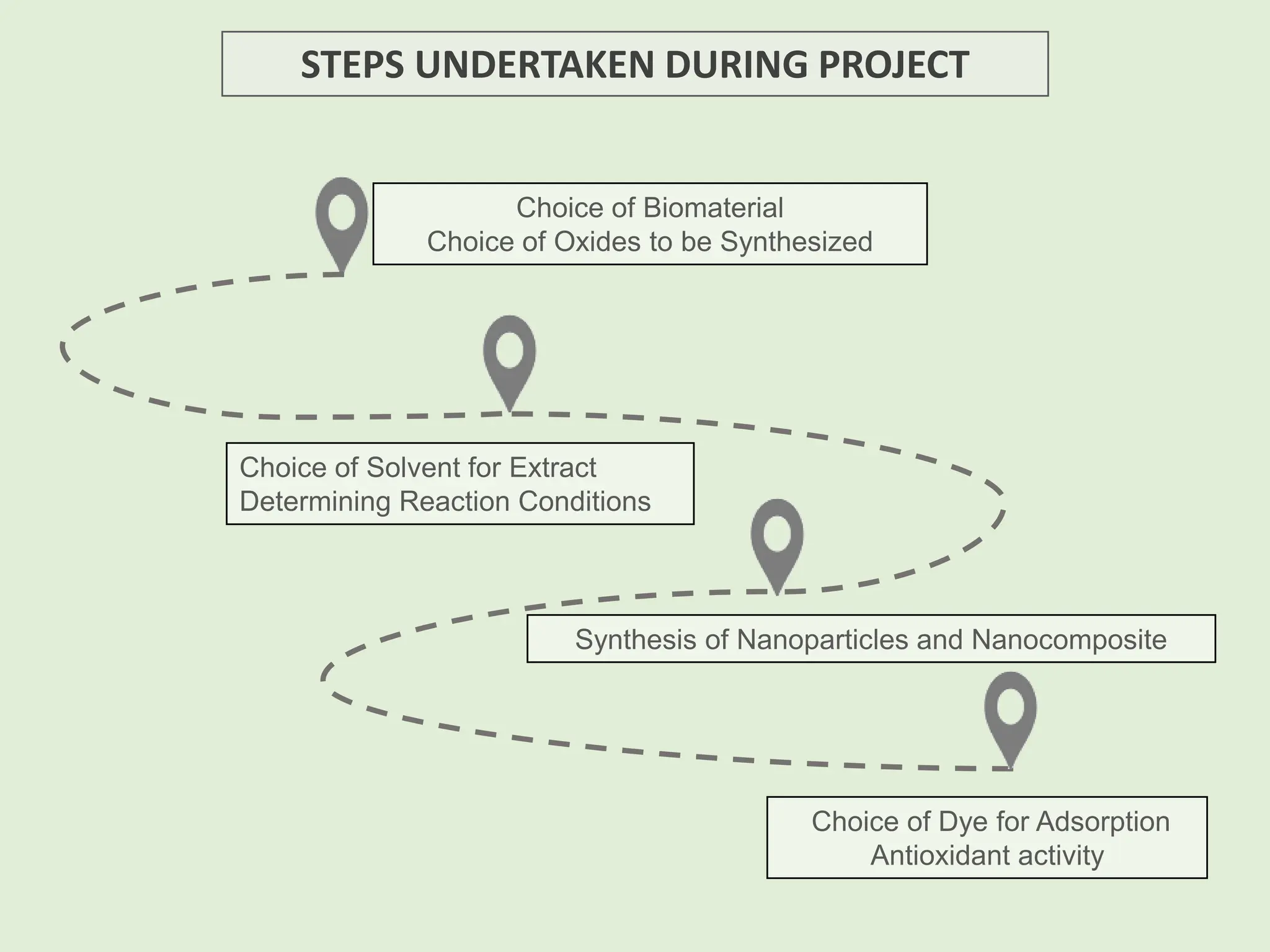
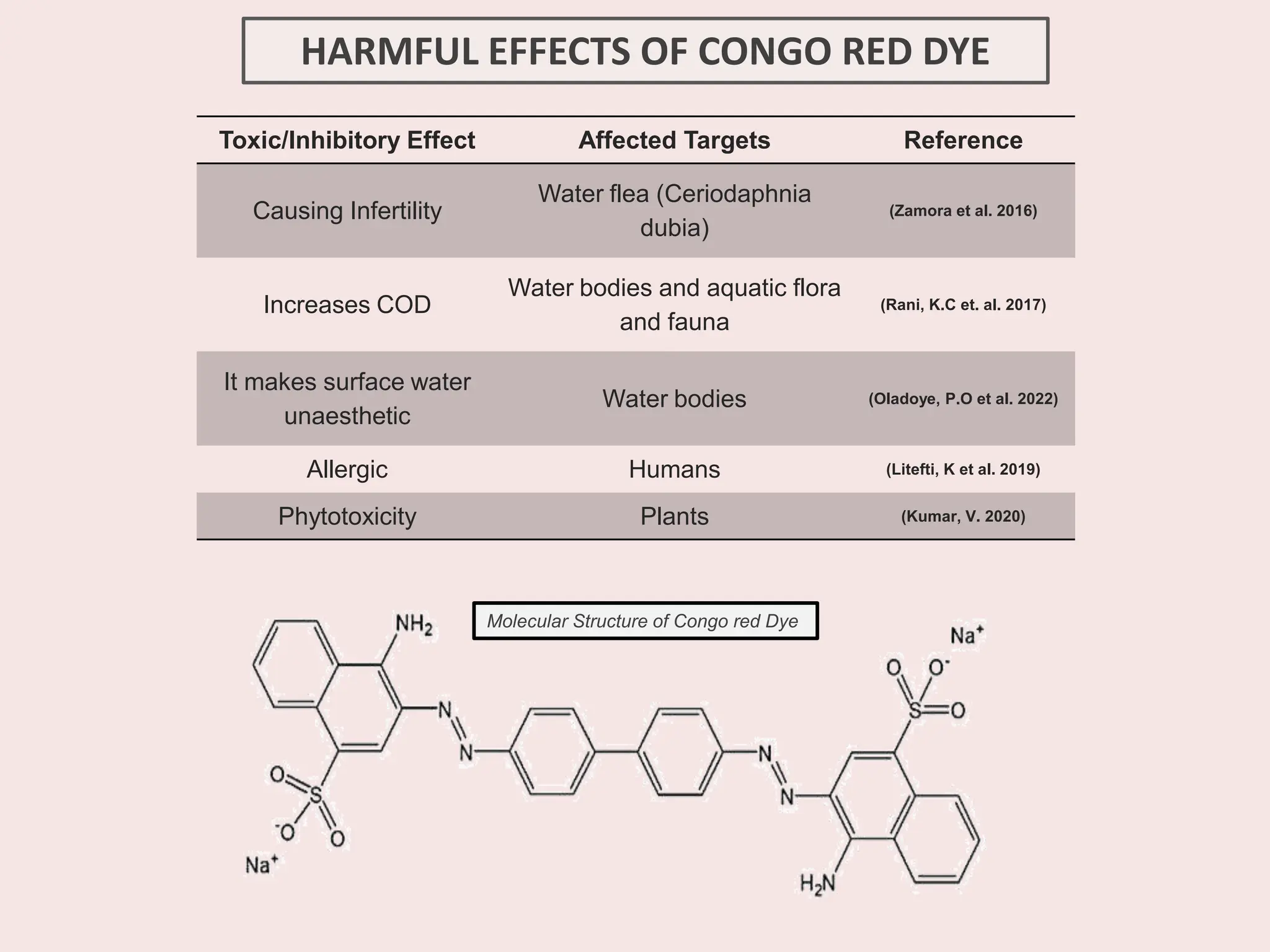
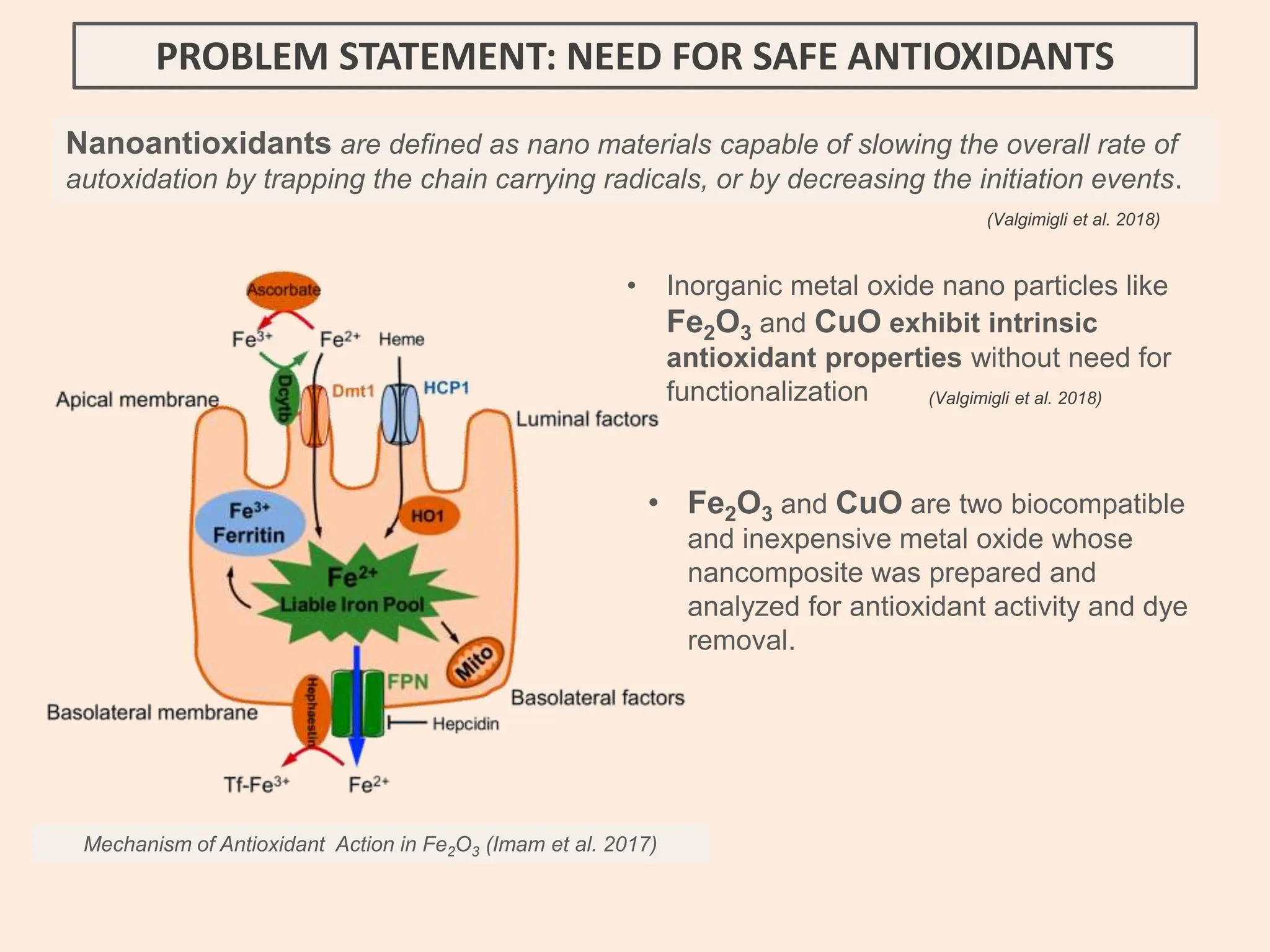
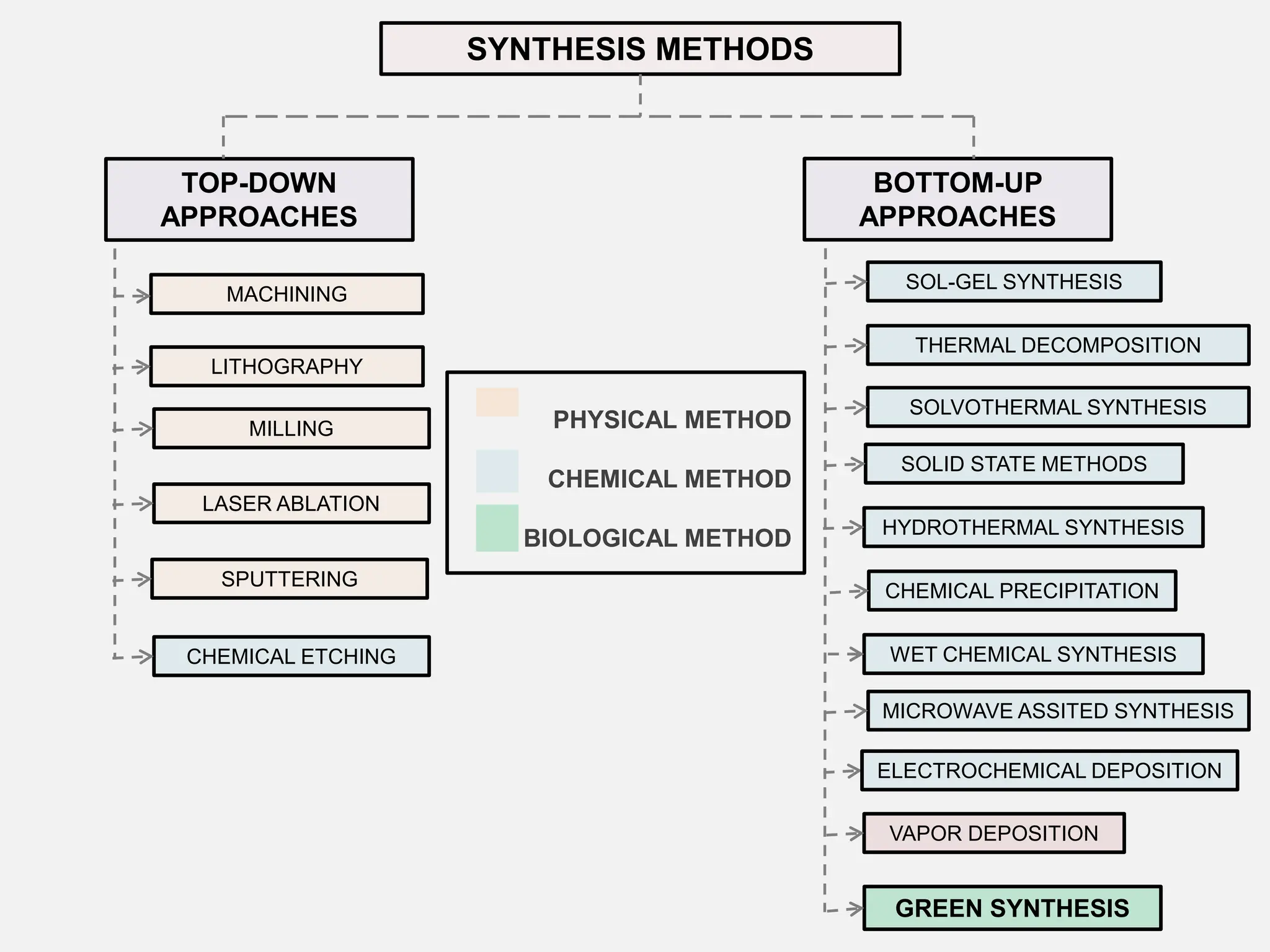
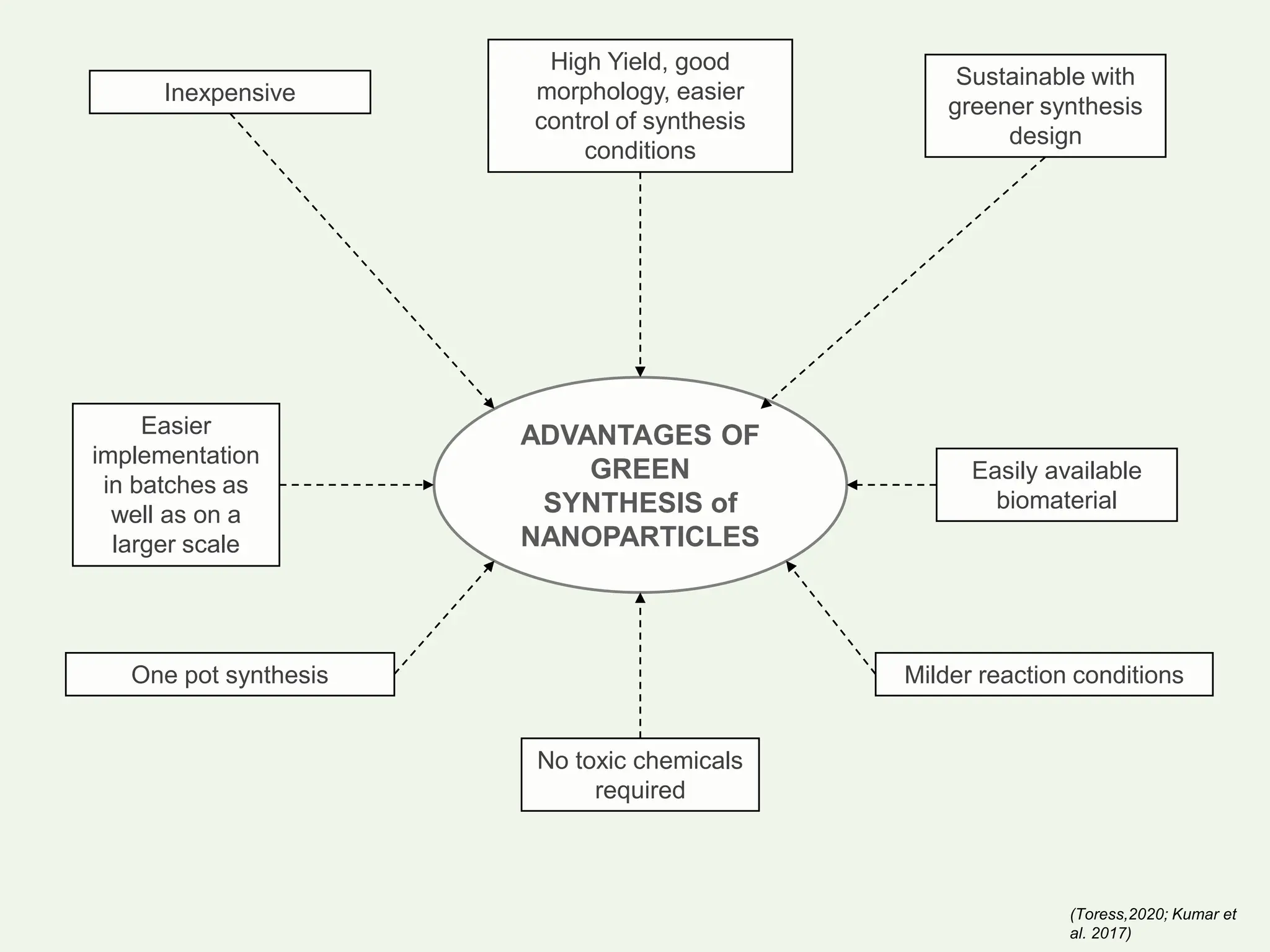
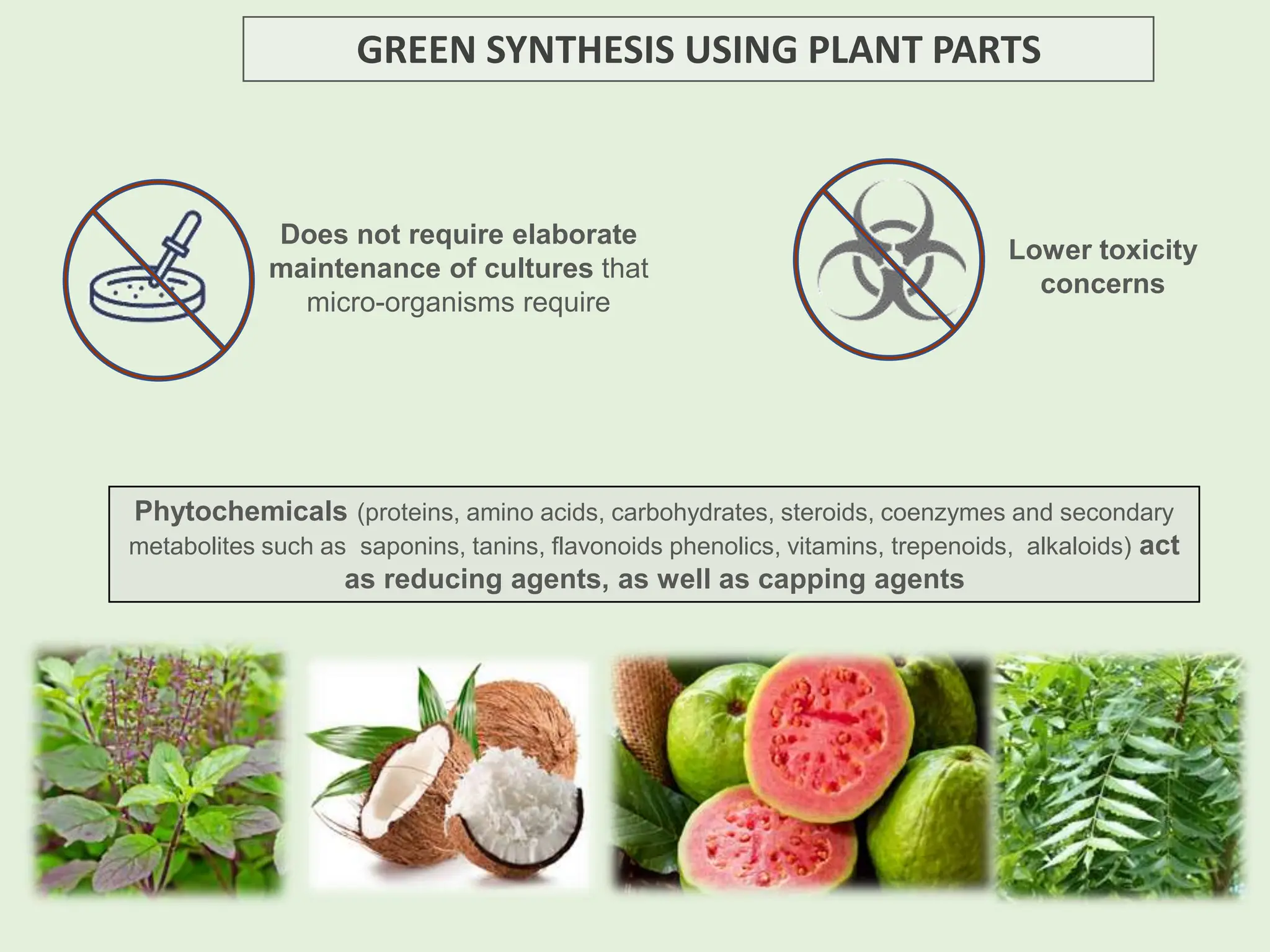
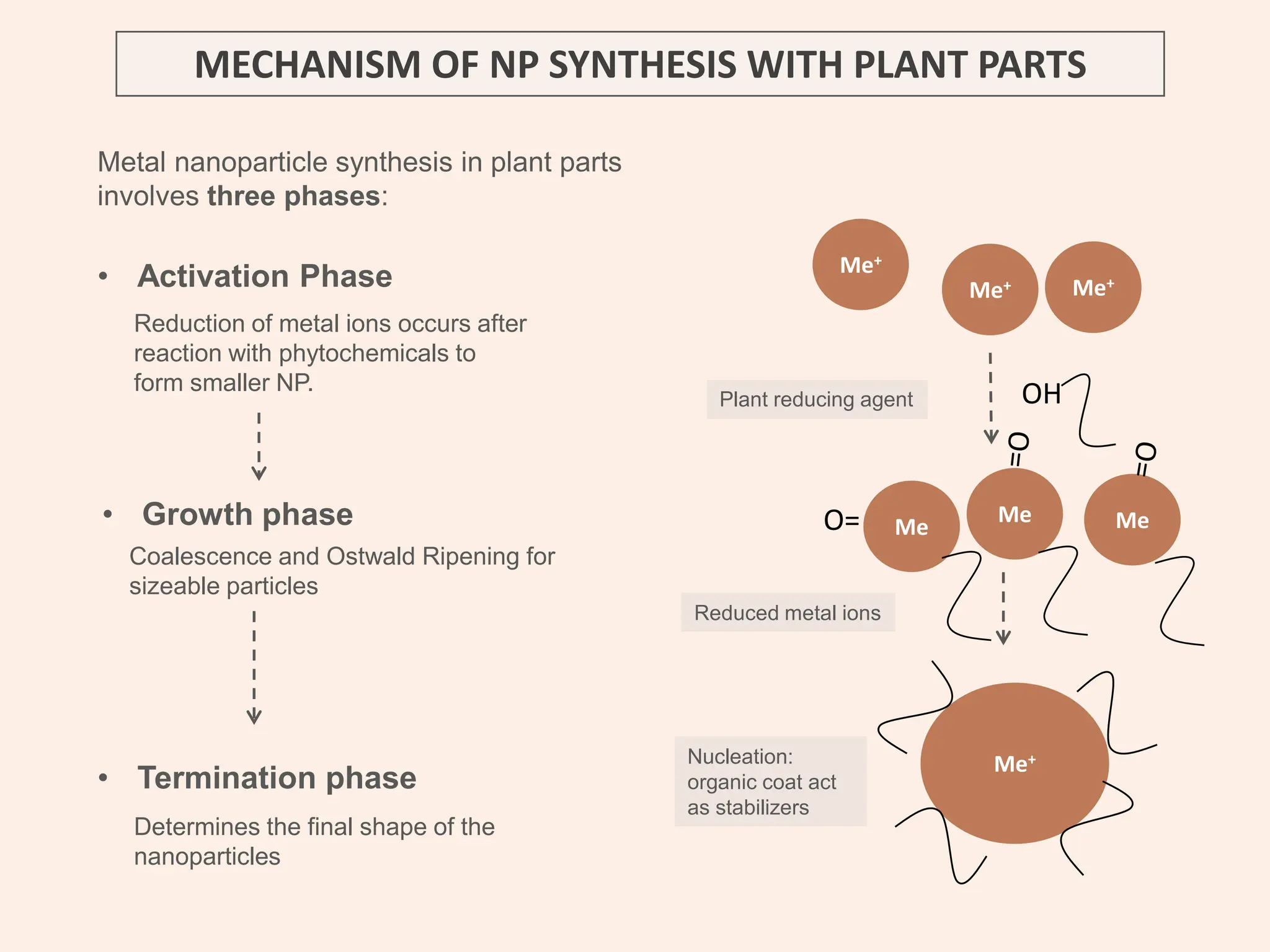
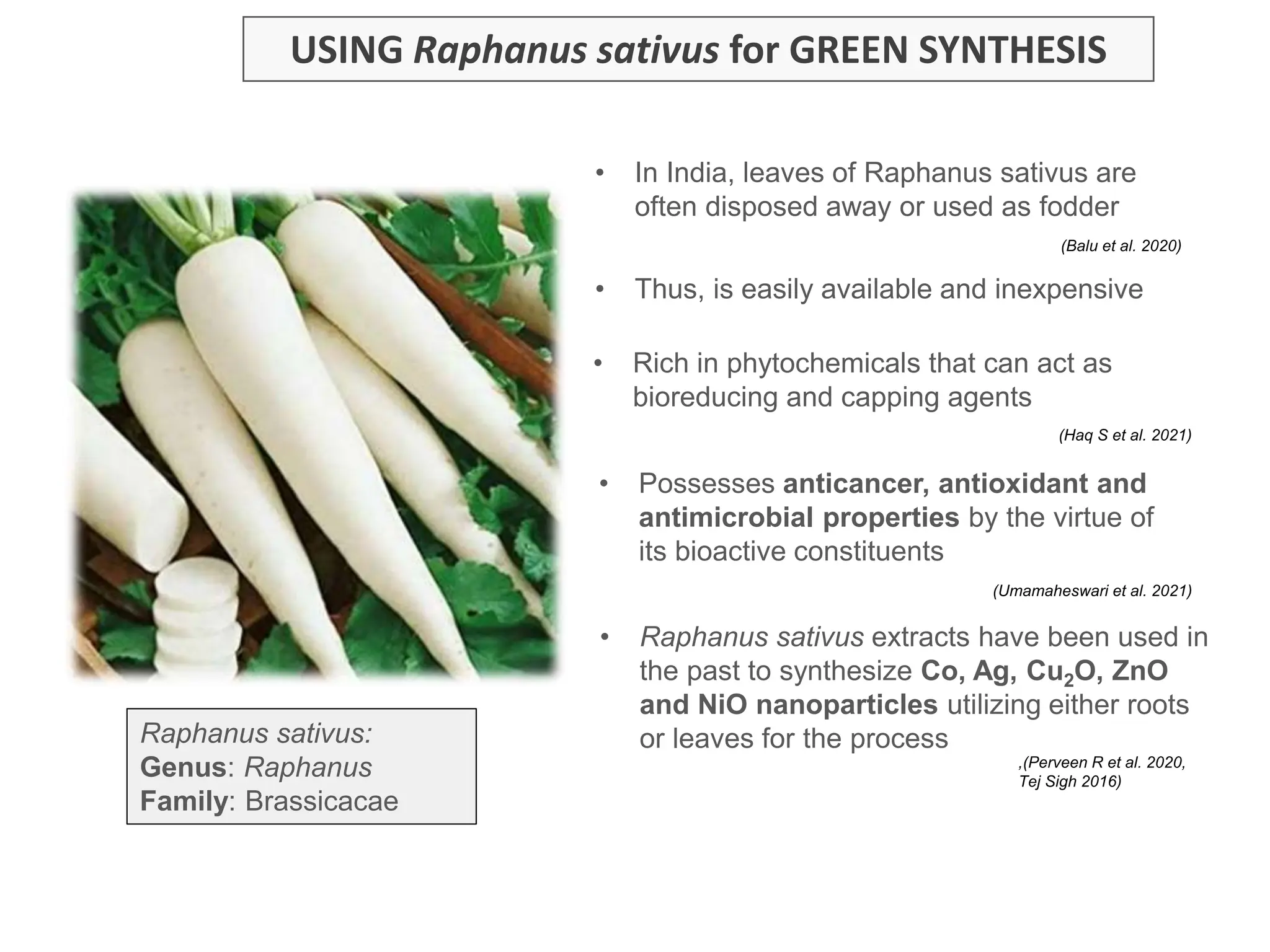
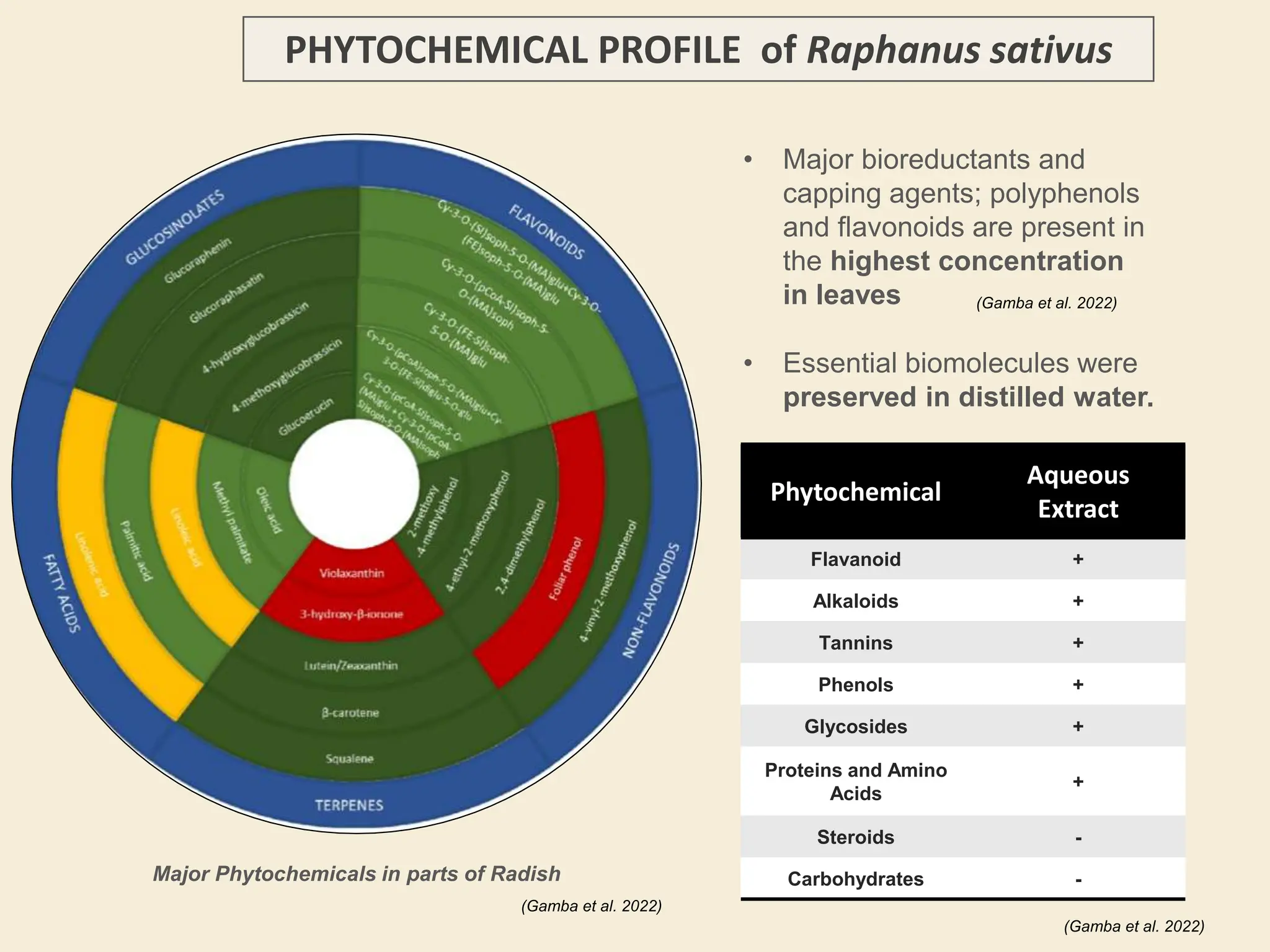
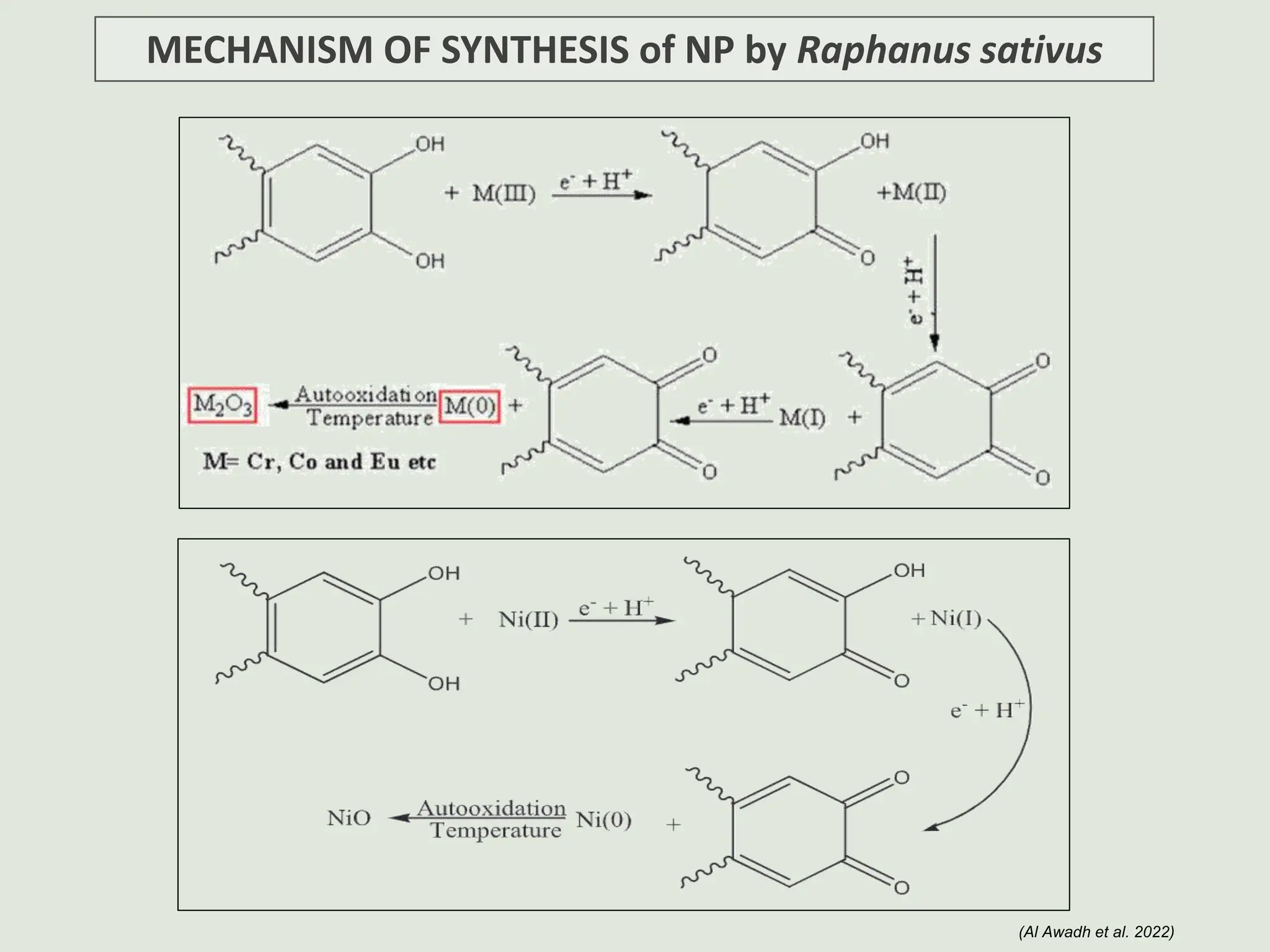
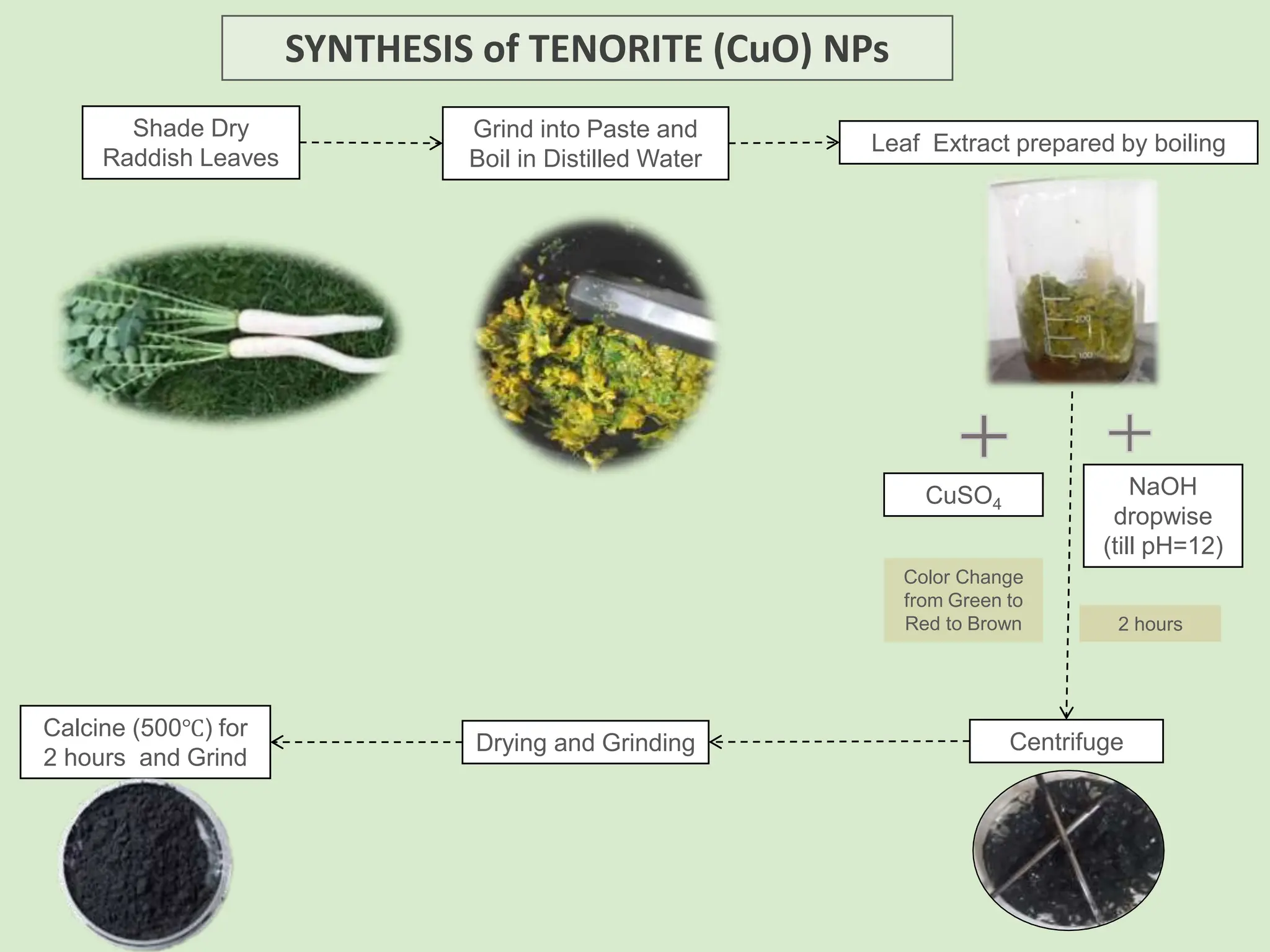
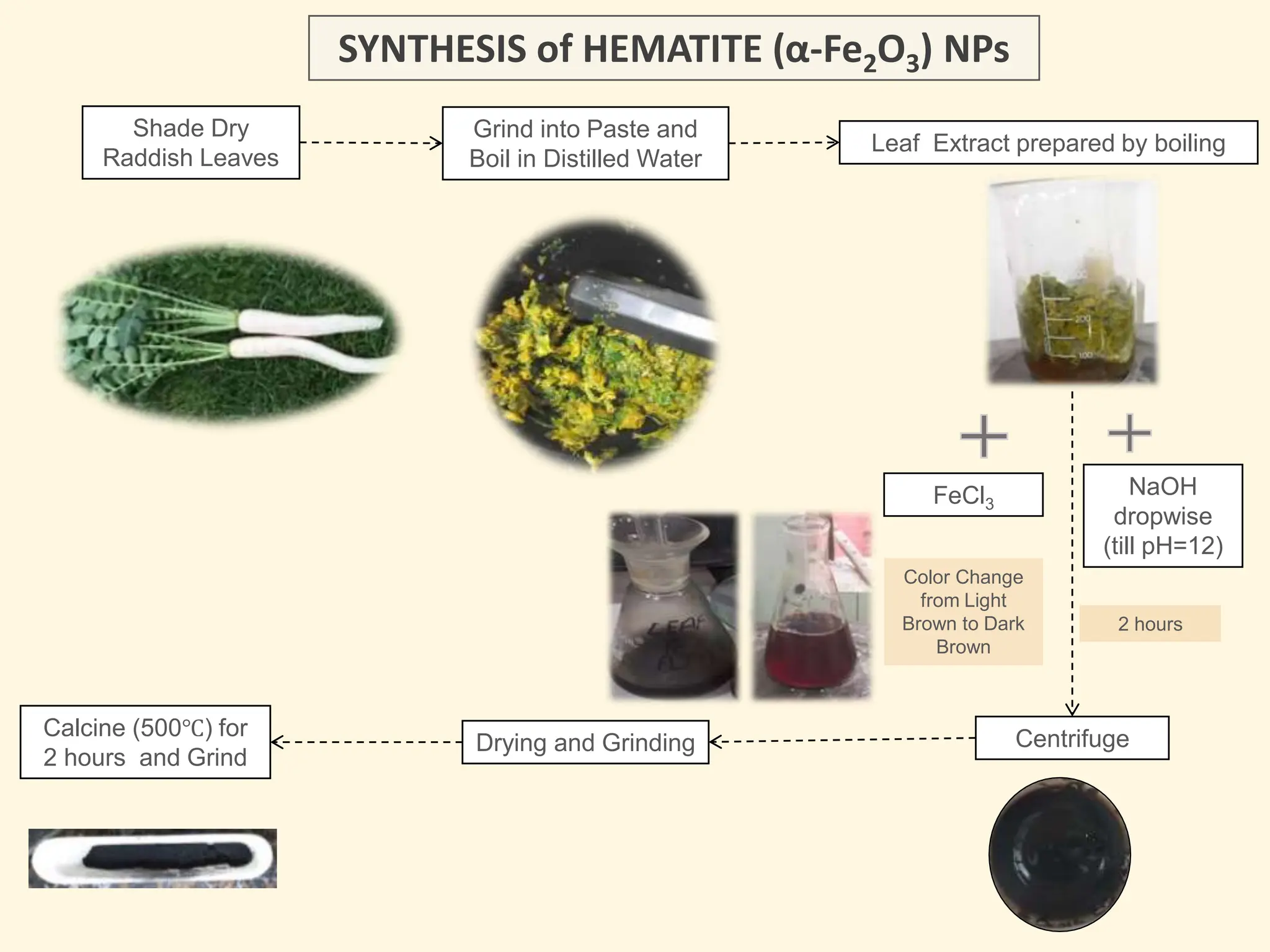
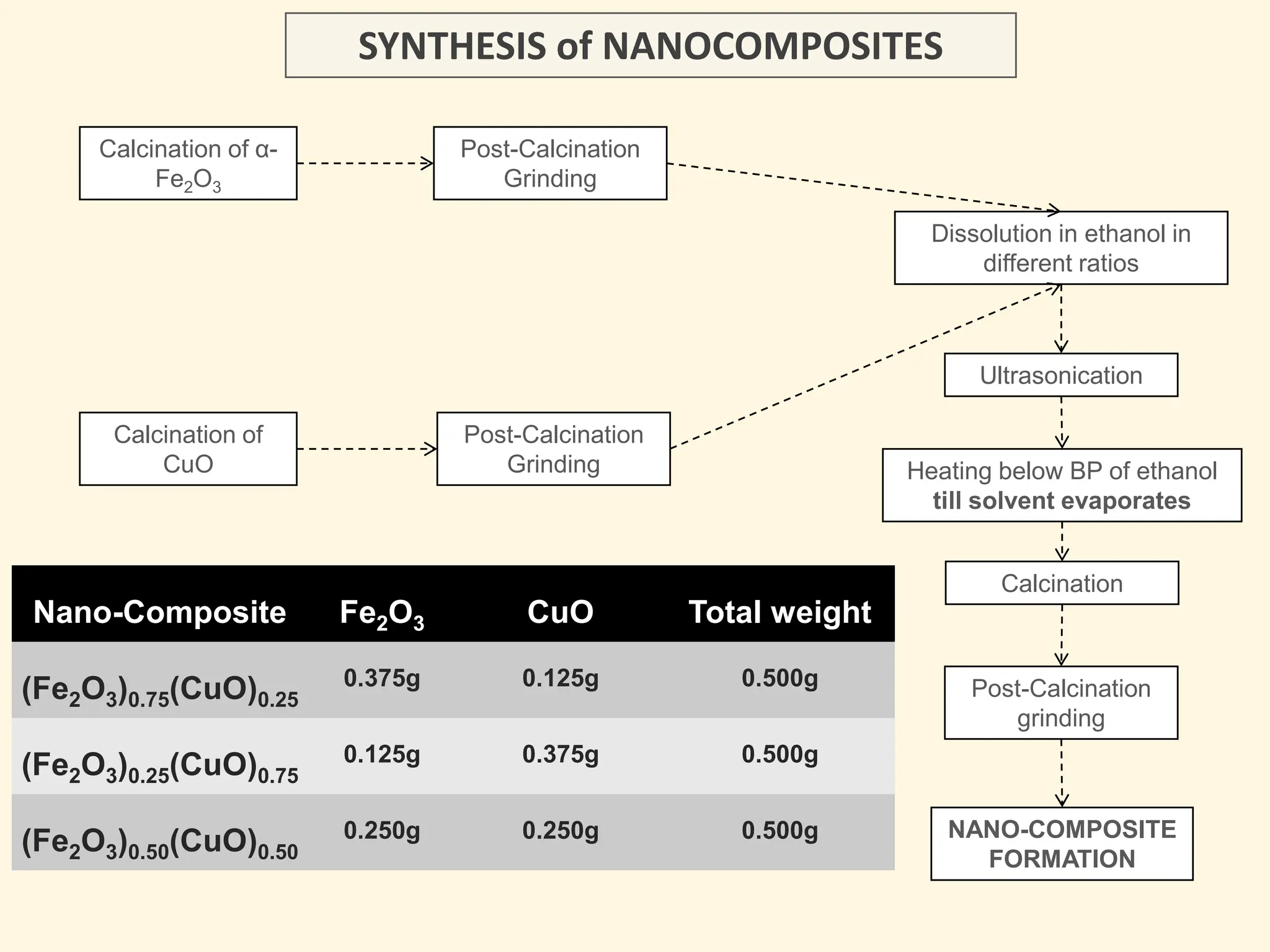
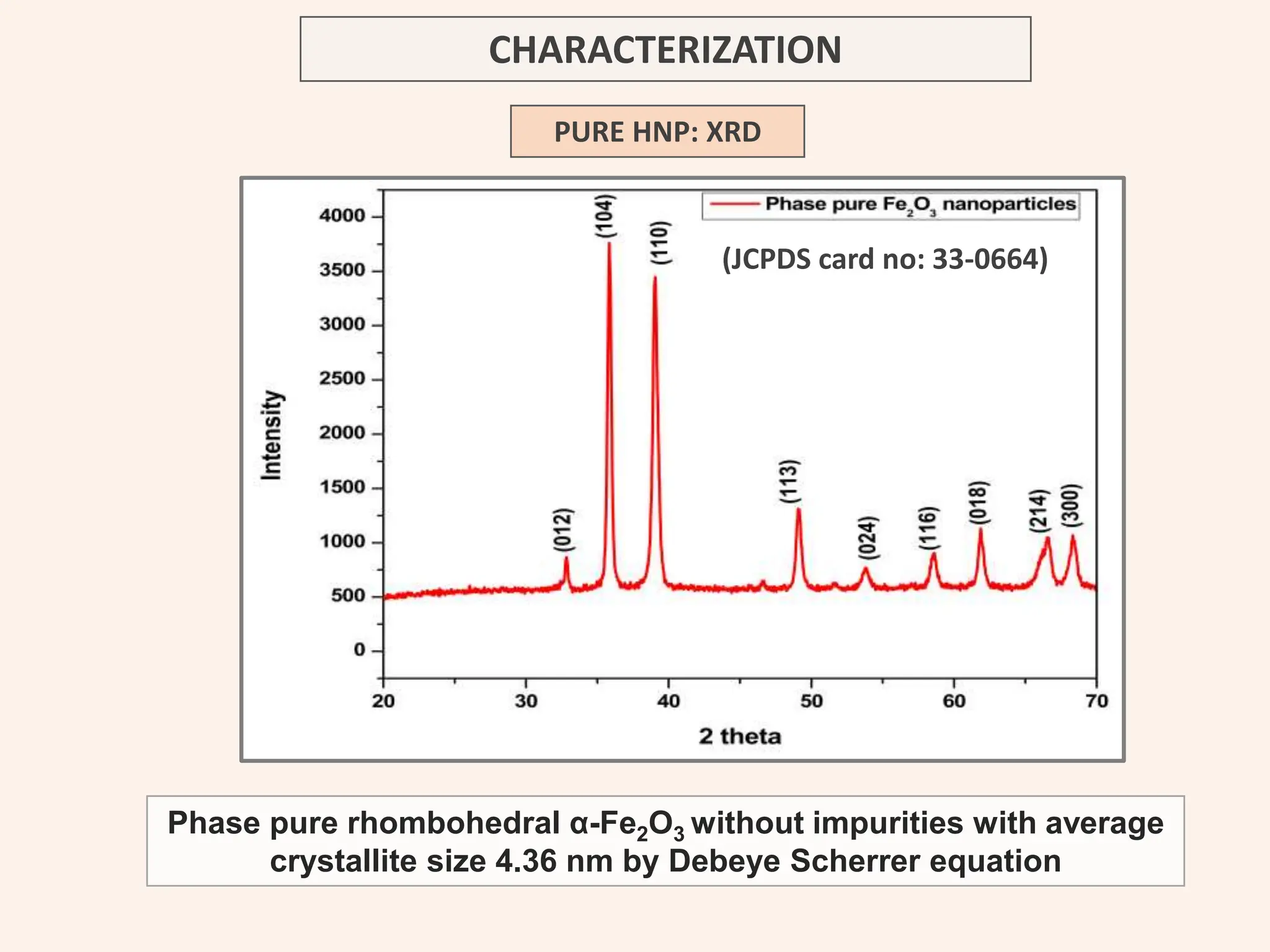
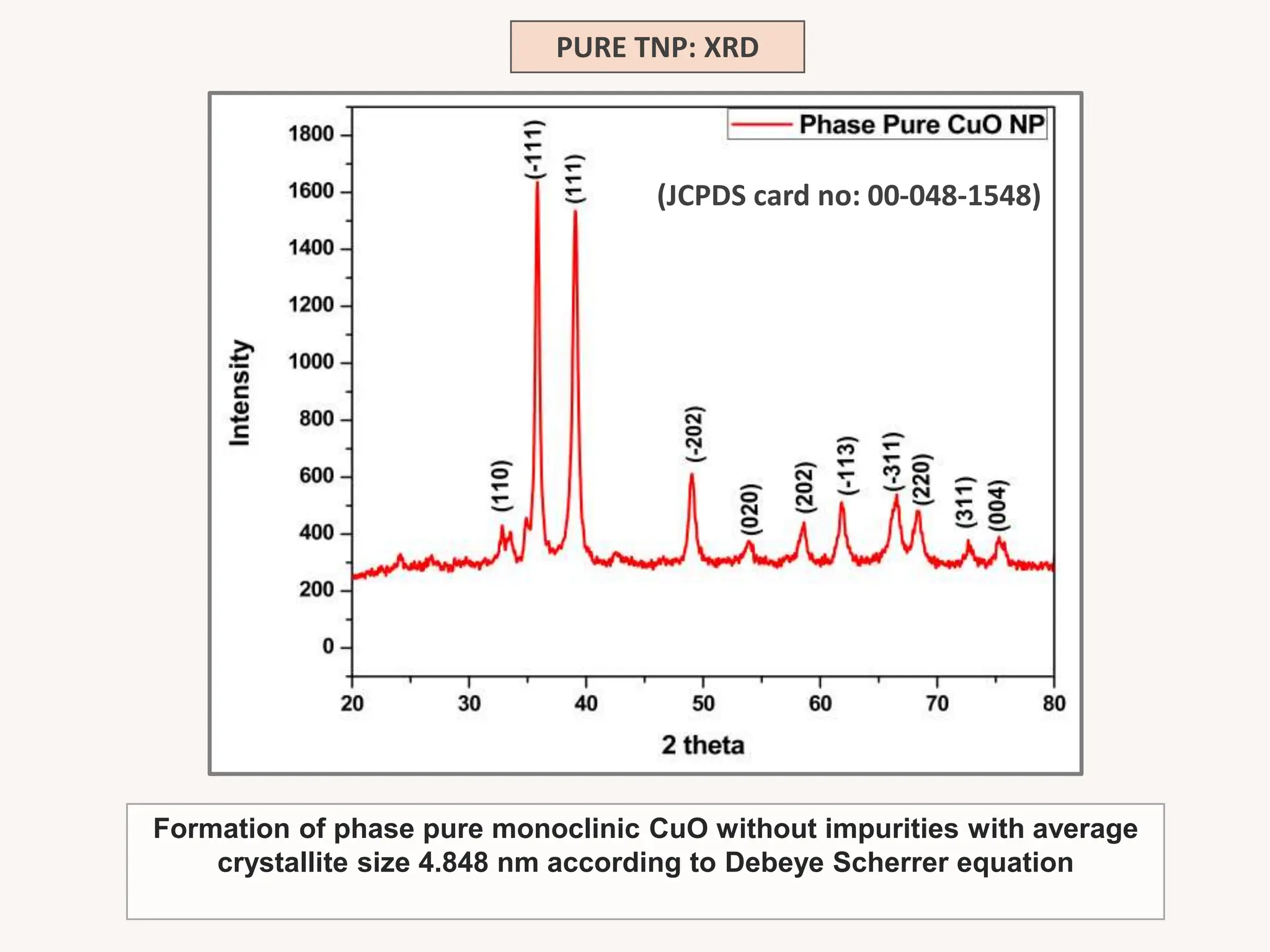
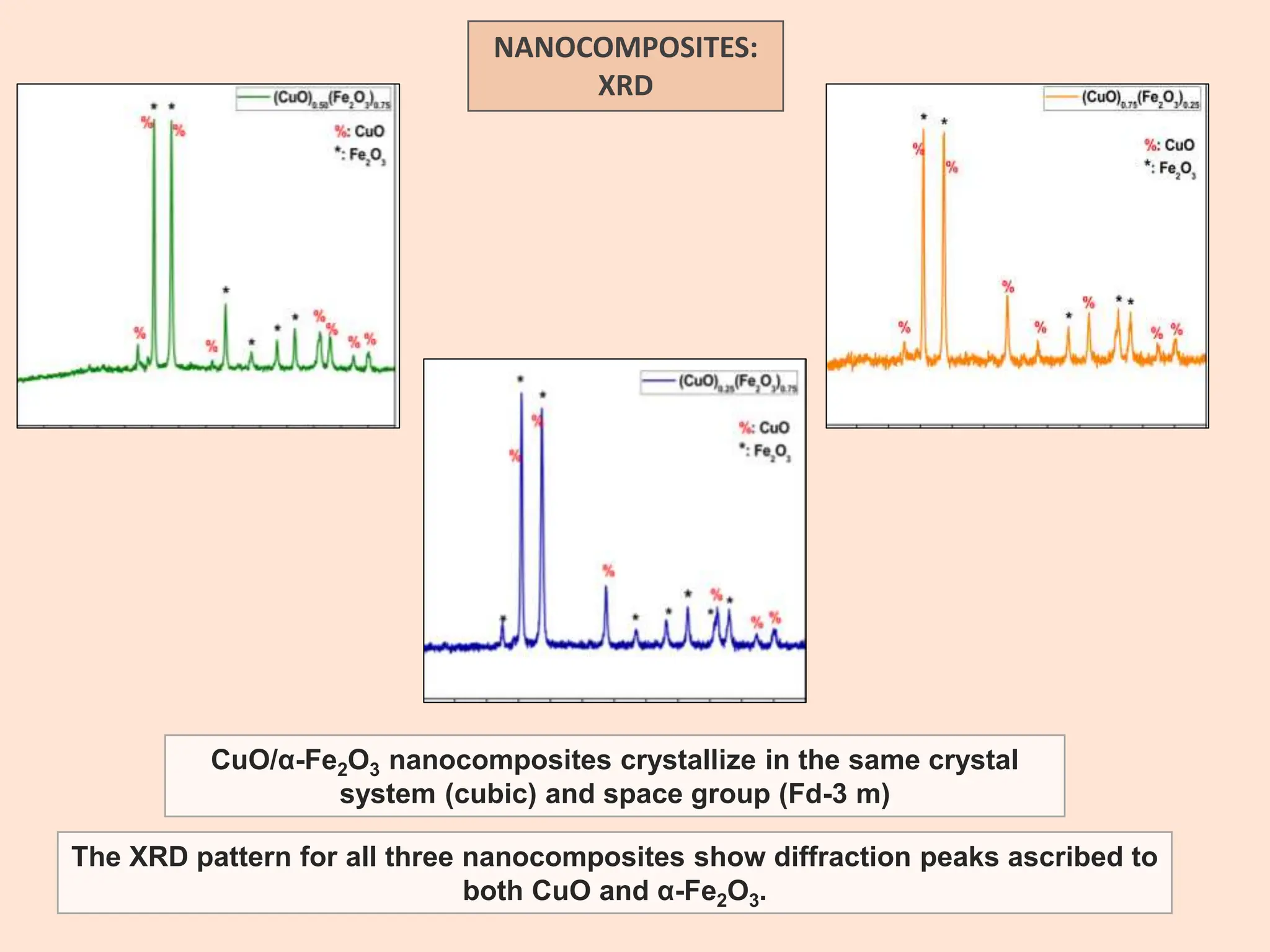
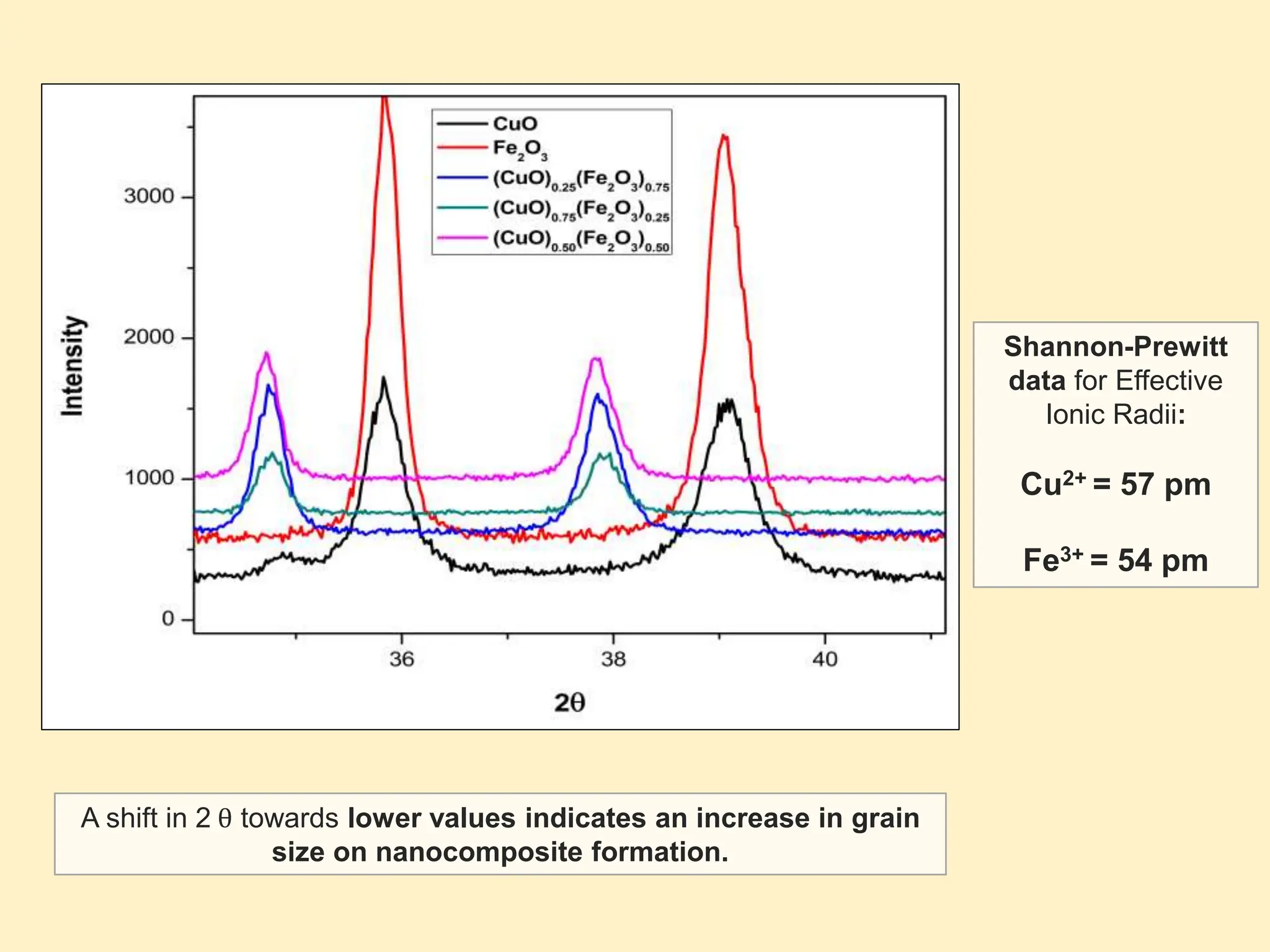
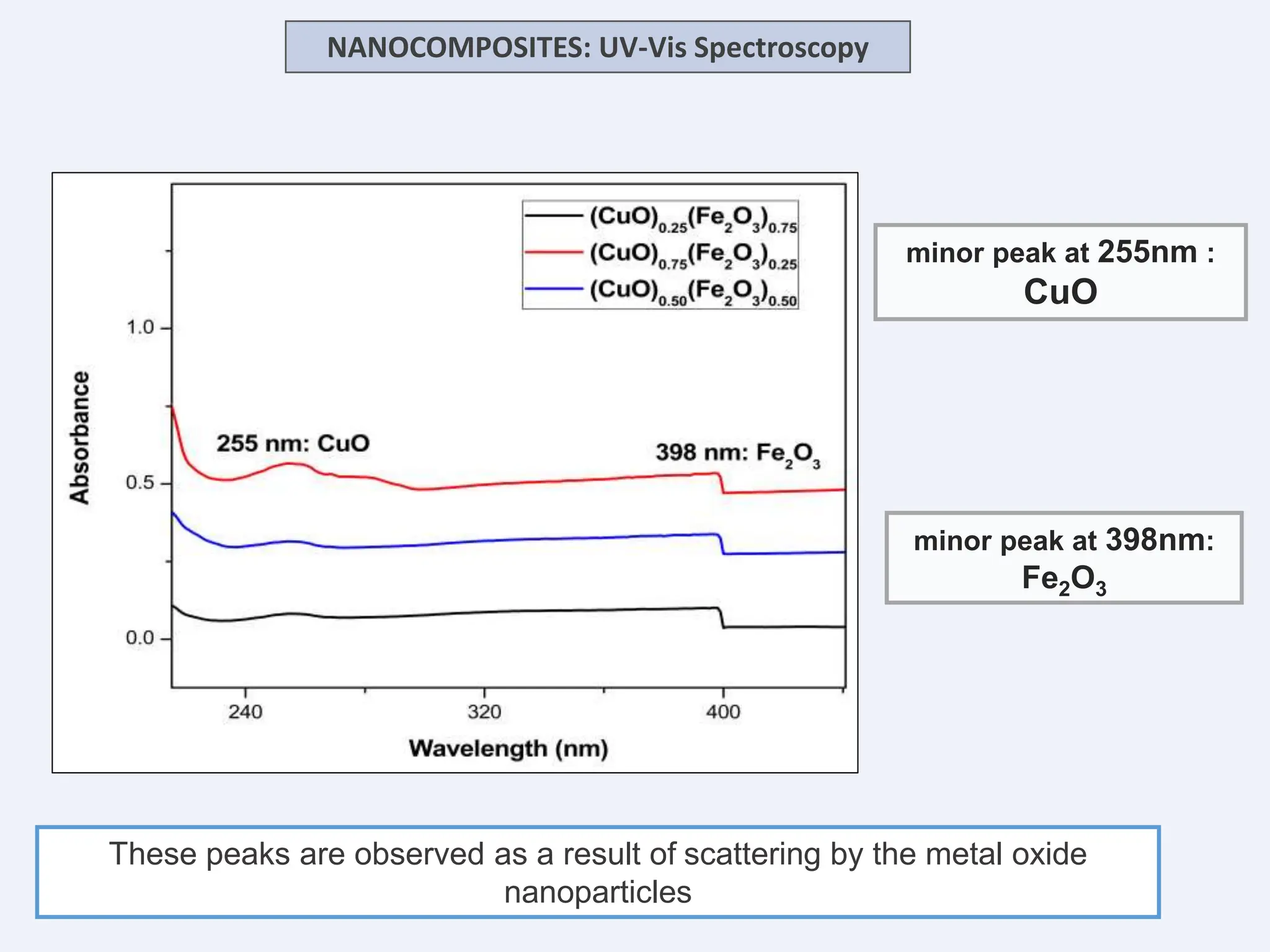
The document discusses the biosynthesis of CuO/Fe2O3 nanocomposites using Raphanus sativus and explores their potential in dye adsorption and antioxidant activities. It highlights the harmful effects of Congo Red dye in water pollution and the advantages of using green synthesis methods with Raphanus sativus due to its rich phytochemical profile. The findings indicate the effectiveness of the synthesized nanocomposites in removing Congo Red dye and suggest their non-toxic application in addressing environmental issues.

![Antioxidant activity was examined through DPPH ASSAY where
absorbance was determined at 517 nm with respect to DPPH
[(Ao - An)/Ao] * 100
(Ao = absorbance of DPPH
; An = absorbance of
DPPH@Nanocomposite
mixture)
.
APPLICATIONS
ANTIOXIDANT APPLICATION
RESULT: values for 0.01mg/ml nanocomposite were calculated to be
83.99%, 72.37% and 91.24%
0
10
20
30
40
50
60
70
80
90
100
25% CuO 75% CuO 50% CuO
%Antioxidant Activity
25% CuO
75% CuO
50% CuO](https://image.slidesharecdn.com/projectpresentation-240609093611-8e934c9d/75/project-presentation-on-nanoparticles-biosynthesis-22-2048.jpg)