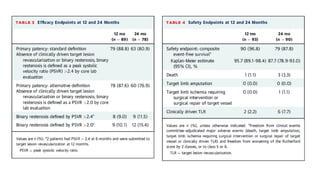
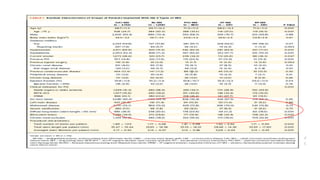
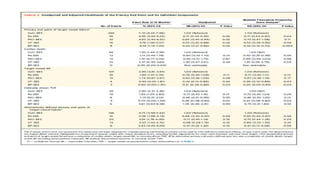
1) The study evaluated 5 types of contemporary drug-eluting stents used in 6,645 patients undergoing complex high-risk procedures. Target vessel failure was lowest with ultrathin sirolimus-eluting stents.
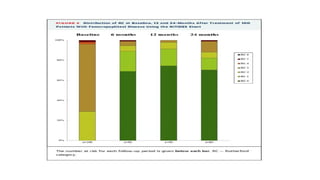
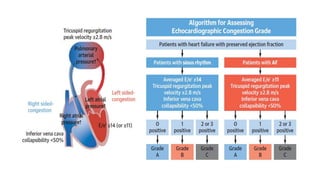
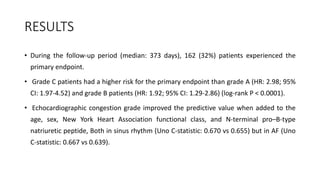
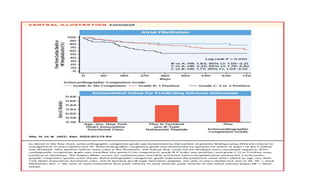
2) Echocardiographic markers of congestion including E/e', tricuspid regurgitation velocity, and inferior vena cava collapsibility predicted outcomes in 505 heart failure patients, both with and without atrial fibrillation. Higher congestion grade was associated with increased risk of cardiovascular death or hospitalization.
3) Right-sided congestion measures may provide prognostic value beyond clinical factors and natriuretic peptides in heart failure with preserved ejection fraction






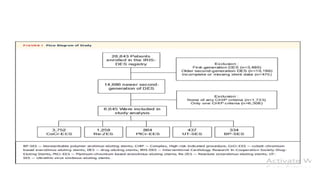
![CHIP
patients with CHIP characteristics were selected in whom both clinical and angiographic criteria had been met (Supplemental
1) clinical criteria of CHIP were 1 of the following characteristics: multiple comorbidities (ie, age >75 years, diabetes mellitus,
chronic renal disease, previous bypass surgery, history of cerebrovascular disease, peripheral artery disease, or chronic lung
disease, ST-segment elevation myocardial infarction (MI) requiring primary PCI, poor ventricular function/hemodynamic
instability (ie, severe left ventricular dysfunction, defined as ejection fraction <30% or clinical presentation with cardiogenic
shock); and
2) angiographic criteria of CHIP were any of the following characteristics: complex coronary lesions (ie, unprotected left main
disease, multivessel disease, severely calcified lesions, very diffuse long lesions [total stent length >40 mm], bifurcation
lesions, or chronic total occlusion).
CHIP patients should have at least 1 clinical criterion as well as at least 1 angiographic criterion.](https://image.slidesharecdn.com/presentation1-230807162715-eadb6639/85/Presentation1-pptx-8-320.jpg)

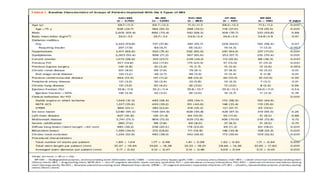
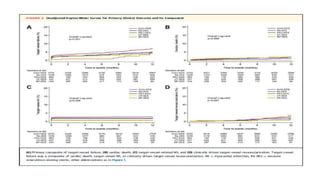
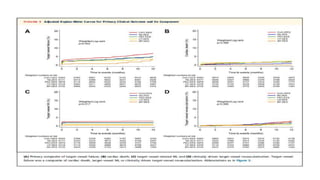
![RESULTS
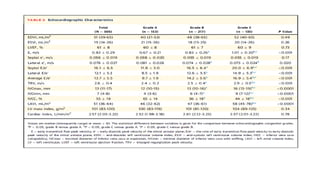
• At 12 months, the rate of target-vessel failure was highest in the CoCr-EES (7.1%)
group; intermediate in the Re-ZES (5.0%), PtCr-EES (4.6%), and BP-SES (4.2%)
groups; and lowest in the UT-SES (3.8%) group (overall long-rank P ¼ 0.001).
• In multiple-treatment propensity-score analysis, the adjusted hazard ratios (HRs)
for target-vessel failure were significantly lower in the Re-ZES (HR: 0.71; 95%
confidence interval [CI]: 0.52-0.97), the UT-SES (HR: 0.52; 95% CI: 0.29-0.95), and
BP-SES (HR: 0.33; 95% CI: 0.16-0.70) groups than in the CoCr-EES group](https://image.slidesharecdn.com/presentation1-230807162715-eadb6639/85/Presentation1-pptx-12-320.jpg)






















![STENT
1. The NiTiDES stent was developed by CID S.p.A. (member of Alvimedica Group).
2. NiTiDES isa CE-certified, polymer-free self-expanding DES in nitinol alloy, loaded with the amphilimus
formulation(sirolimus plus fatty acid) enhancing drug bioavailability .
3. The drug is contained within grooves (reservoirs) on the outer surface of the stent (Abluminal Reservoir
Technology), and therefore it is eluted only toward the vessel wall, allowing a fast stent re-
endothelialization.
4. The entire structure, including the reservoirs, is homogeneously coated with an ultra-thin film of pure
carbon (i-Carbofilm [Bio Inducer Surface]) in order to increase hemocompatibility and biocompatibility
and ensure thromboresistance.](https://image.slidesharecdn.com/presentation1-230807162715-eadb6639/85/Presentation1-pptx-55-320.jpg)

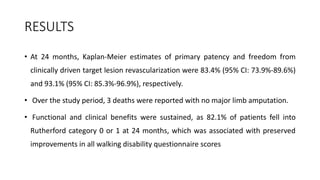
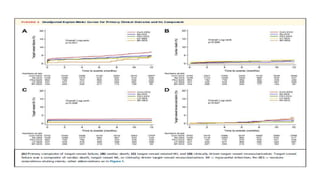
![1. The observed primary patency rate of 83.4% in the ILLUMINA studycompares well with other trials investigating DES for
femoropopliteal interventions.
2. In the recently published IMPERIAL (A Randomized Trial Comparing the ELUVIA Drug-eluting Stent Versus Zilver PTX
Stent for Treatment of Superficial Femoral and/or Proximal Popliteal Arteries) trial comparing 2 paclitaxel-eluting stents,
one with a polymer coating (Eluvia [Boston Scientific]) and one without (Zilver PTX [Cook Medical]), the primary patency
rate was 83.0% for Eluvia and 77.1% for Zilver PTX after 24 months of follow-up.
3. In the BATTLE (Bare Metal Stent vs. Paclitaxel Eluting Stent in the Setting of Primary Stenting of Intermediate-Length
Femoropopliteal Lesions) trial, a head-to-head randomized comparison of the Zilver PTX vs a BMS, patency rates of
78.8% and 74.6% were reported at 2 years for the DES and BMS, respectively.15](https://image.slidesharecdn.com/presentation1-230807162715-eadb6639/85/Presentation1-pptx-58-320.jpg)