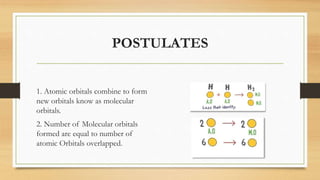
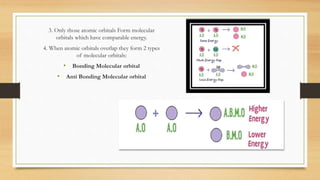
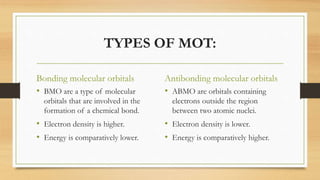
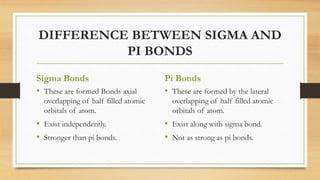
This document provides an overview of molecular orbital theory (MOT), including its postulates, types of molecular orbitals, and the differences between sigma and pi bonds. It explains the formation of bonding and antibonding molecular orbitals, their energy levels, and the concept of linear combination of atomic orbitals (LCAO). The key concepts highlighted are the relation between atomic and molecular orbitals and the strength variations between sigma and pi bonds.