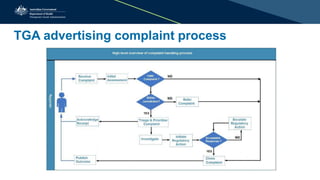
The document outlines the key points of the new Advertising Code effective from January 1, 2019, which requires compliance with therapeutic goods advertising standards to prevent misleading consumers. Major differences from the 2015 code include clearer requirements for information in advertising and new rules surrounding testimonials and endorsements, as well as guidelines for handling advertising complaints. It emphasizes the responsibility of advertisers to ensure compliance and provides resources for support, aiming to promote responsible advertising and protect public health.