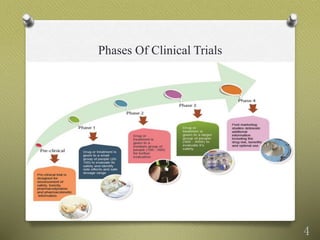
This document discusses problems with evidence from clinical drug studies and why such evidence may fail to accurately predict a drug's clinical utility. Some key issues include: insufficient consideration of all available evidence; use of weak study designs like non-randomized trials; industry sponsorship resulting in bias; lack of head-to-head comparisons; choice of populations and outcomes that do not reflect real-world use; publication and reporting biases that suppress negative results; and reliability issues with meta-analyses and clinical guidelines formed from potentially biased evidence. Solutions proposed include improving study registration and data sharing, requiring comparative evidence earlier, and conducting reviews in a more transparent and non-conflicted manner.