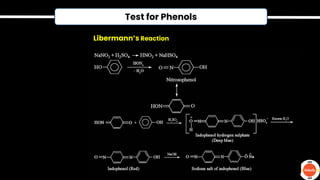
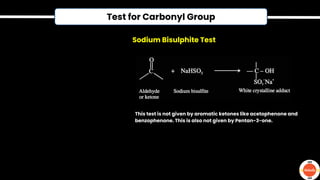
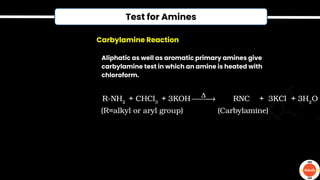
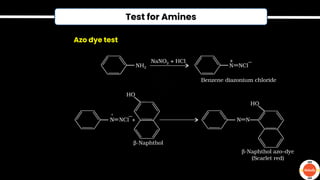
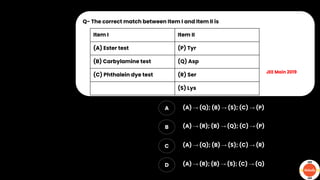
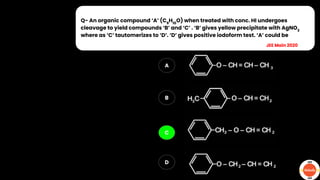
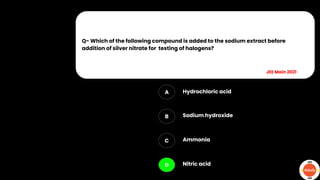
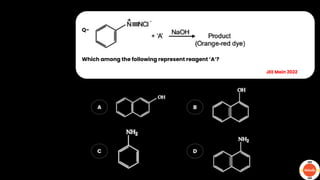
The document outlines various practical tests in organic chemistry for detecting elements and functional groups, including tests for carbon, hydrogen, nitrogen, sulfur, and phosphorus. It describes specific reactions such as Liebig's test, the 2,4-dinitrophenylhydrazine test for carbonyl groups, and the biuret test for proteins, among others. Additionally, it includes information on distinguishing different types of carbohydrates and amino acids through various chemical reactions and tests, providing a comprehensive overview of qualitative analysis methods.





























































![Q- A chemist has 4 samples of artificial sweetener A, B, C and D. To identify these
samples, he performed certain experiments and noted the following observations :
(i) A and D both form blue-violet colour with ninhydrin.
(ii) Lassaigne extract of C gives positive AgNO3
test and negative Fe4
[Fe(CN)6
]3
test.
(iii) Lassaigne extract of B and D gives positive sodium nitroprusside test.
Based on these observations which option is correct?
A
B
C
D
A : Aspartame; B : Alitame; C : Saccharin; D : Sucralose
A : Saccharin; B : Alitame; C : Sucralose; D : Aspartame
A : Alitame; B : Saccharin; C : Aspartame; D : Sucralose
A : Aspartame; B : Saccharin; C : Sucralose; D : Alitame
JEE Main 2020](https://image.slidesharecdn.com/practicalorganicchemistry-240908055558-5f9dad37/85/Practical-Organic-Chemistry-problems-for-Jee-68-320.jpg)
![Q- A chemist has 4 samples of artificial sweetener A, B, C and D. To identify these
samples, he performed certain experiments and noted the following observations :
(i) A and D both form blue-violet colour with ninhydrin.
(ii) Lassaigne extract of C gives positive AgNO3
test and negative Fe4
[Fe(CN)6
]3
test.
(iii) Lassaigne extract of B and D gives positive sodium nitroprusside test.
Based on these observations which option is correct?
A
B
C
D
A : Aspartame; B : Alitame; C : Saccharin; D : Sucralose
A : Saccharin; B : Alitame; C : Sucralose; D : Aspartame
A : Alitame; B : Saccharin; C : Aspartame; D : Sucralose
A : Aspartame; B : Saccharin; C : Sucralose; D : Alitame
JEE Main 2020](https://image.slidesharecdn.com/practicalorganicchemistry-240908055558-5f9dad37/85/Practical-Organic-Chemistry-problems-for-Jee-69-320.jpg)











![Q- In Tollen's test for aldehyde, the overall number of electron(s) transferred to the
Tollen's reagent formula [Ag(NH3
)2
]+
per aldehyde group to form silver mirror is
____.
(Round off to the Nearest Integer).
JEE Main 2021](https://image.slidesharecdn.com/practicalorganicchemistry-240908055558-5f9dad37/85/Practical-Organic-Chemistry-problems-for-Jee-81-320.jpg)
![Q- In Tollen's test for aldehyde, the overall number of electron(s) transferred to the
Tollen's reagent formula [Ag(NH3
)2
]+
per aldehyde group to form silver mirror is
____.
(Round off to the Nearest Integer).
JEE Main 2021
Ans 2](https://image.slidesharecdn.com/practicalorganicchemistry-240908055558-5f9dad37/85/Practical-Organic-Chemistry-problems-for-Jee-82-320.jpg)

















![Q- The formula of the purple colour formed in Lassaigne's test for sulphur using
sodium nitroprusside is
A
B
C
D
NaFe[Fe(CN)6
]
Na[Cr(NH3
)2
(NCS)4
]
Na2
[Fe(CN)5
(NO)]
Na4
[Fe(CN5
)(NOS)]
JEE Main 2022](https://image.slidesharecdn.com/practicalorganicchemistry-240908055558-5f9dad37/85/Practical-Organic-Chemistry-problems-for-Jee-102-320.jpg)
![Q- The formula of the purple colour formed in Lassaigne's test for sulphur using
sodium nitroprusside is
A
B
C
D
NaFe[Fe(CN)6
]
Na[Cr(NH3
)2
(NCS)4
]
Na2
[Fe(CN)5
(NO)]
Na4
[Fe(CN5
)(NOS)]
JEE Main 2022](https://image.slidesharecdn.com/practicalorganicchemistry-240908055558-5f9dad37/85/Practical-Organic-Chemistry-problems-for-Jee-103-320.jpg)
![Solution:
Purple
S2-
+ [Fe(CN)5
NO]2-
→ [Fe(CN)5
(NOS)]4-](https://image.slidesharecdn.com/practicalorganicchemistry-240908055558-5f9dad37/85/Practical-Organic-Chemistry-problems-for-Jee-104-320.jpg)


