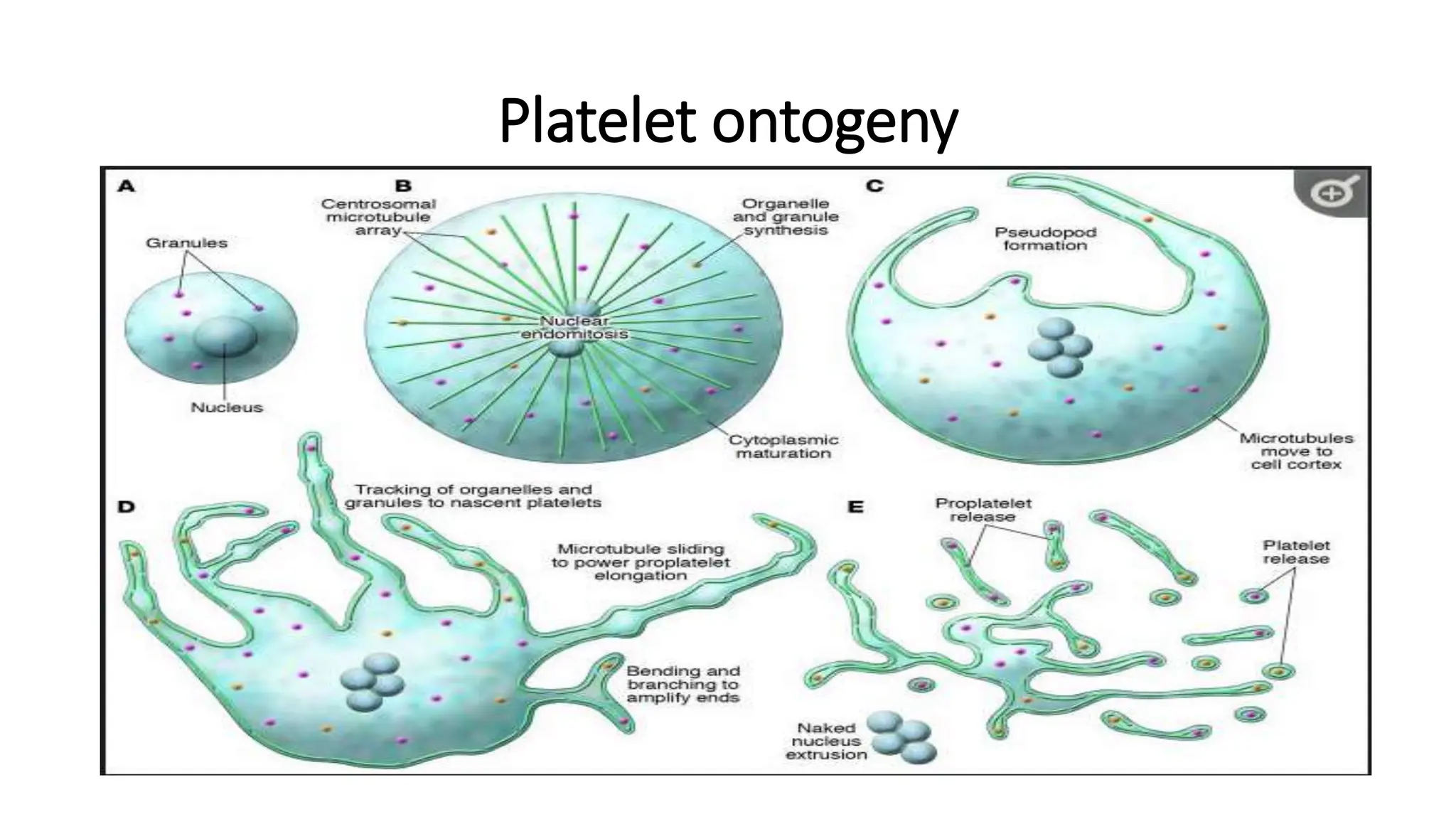
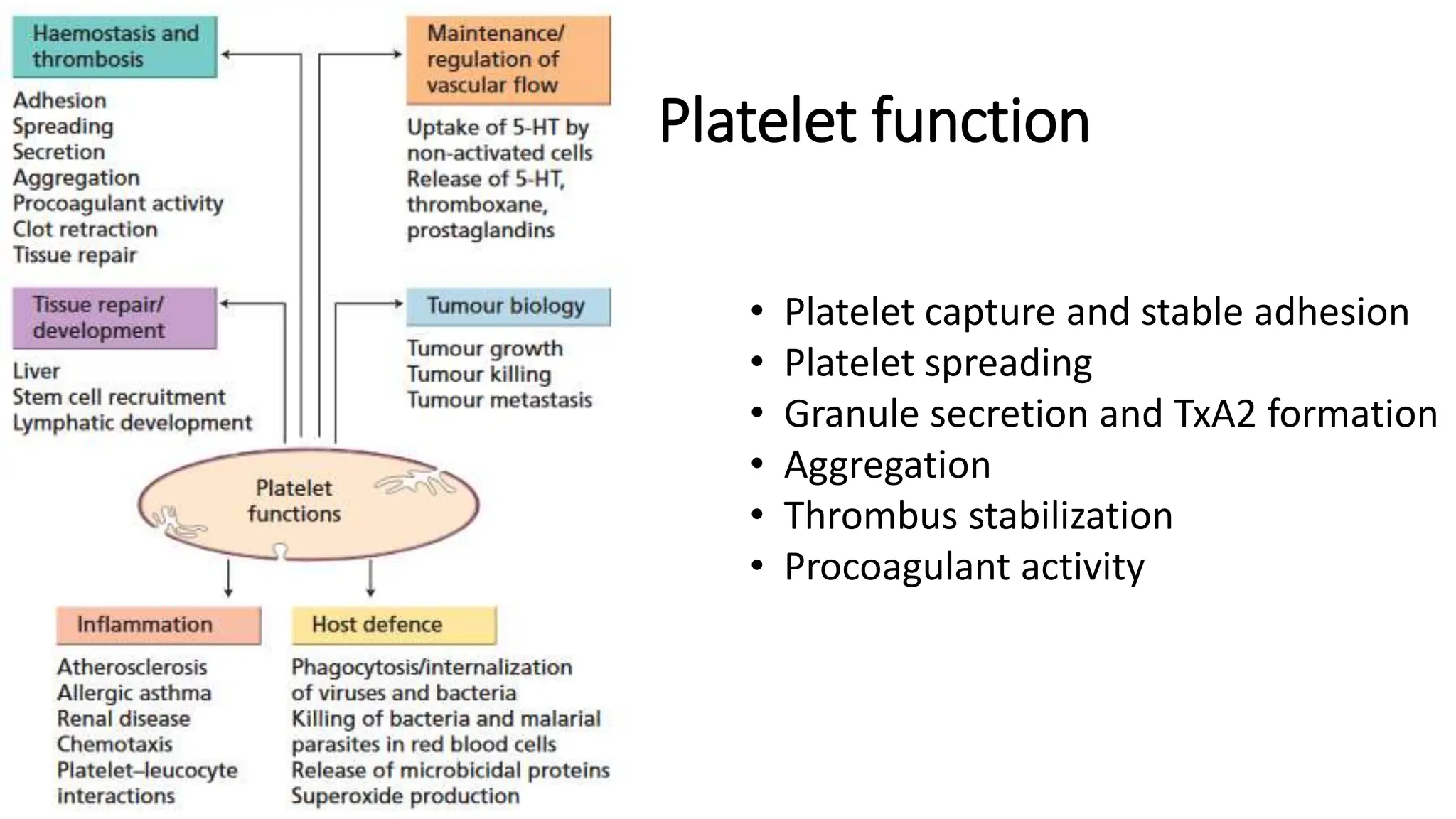
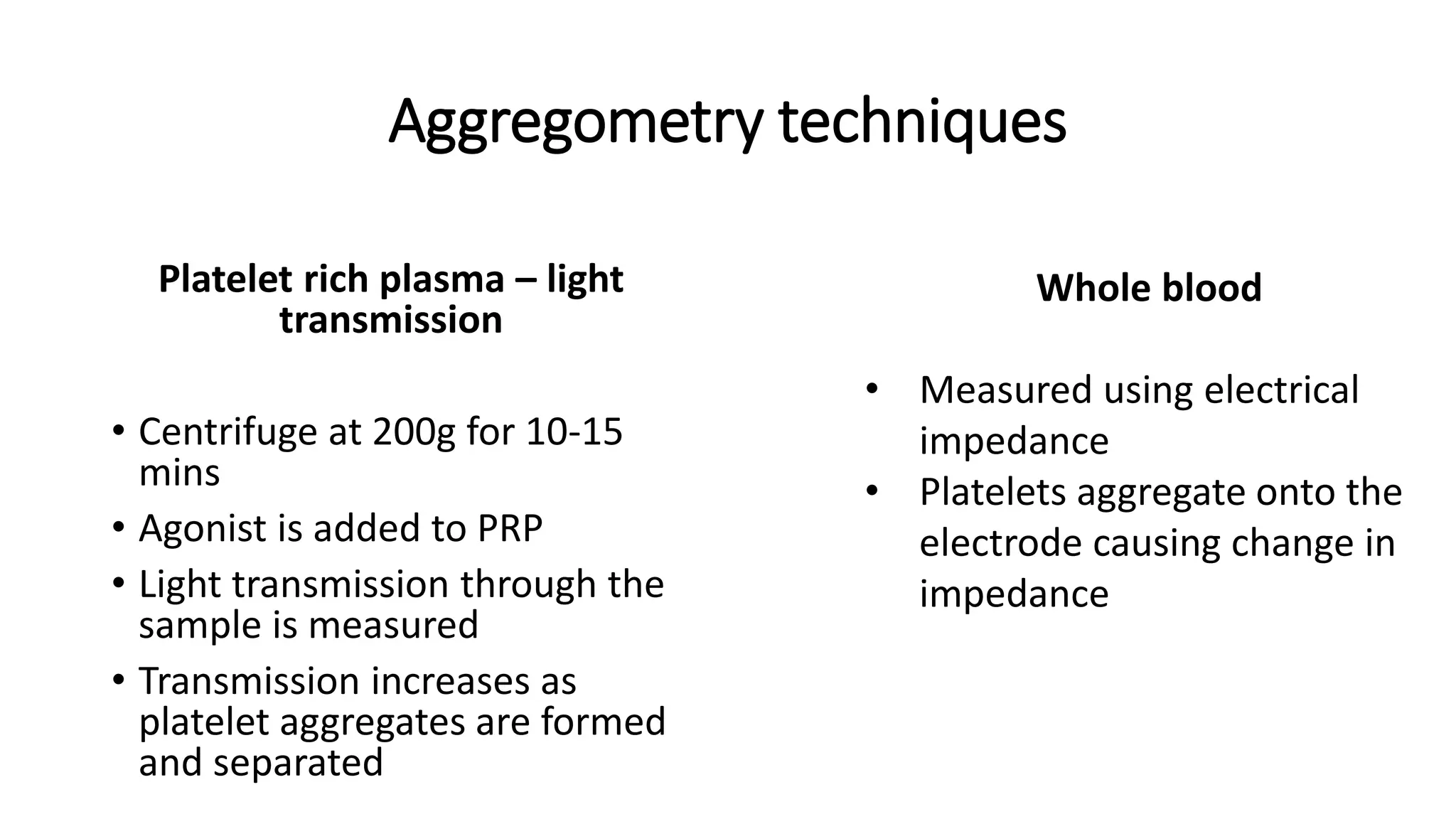
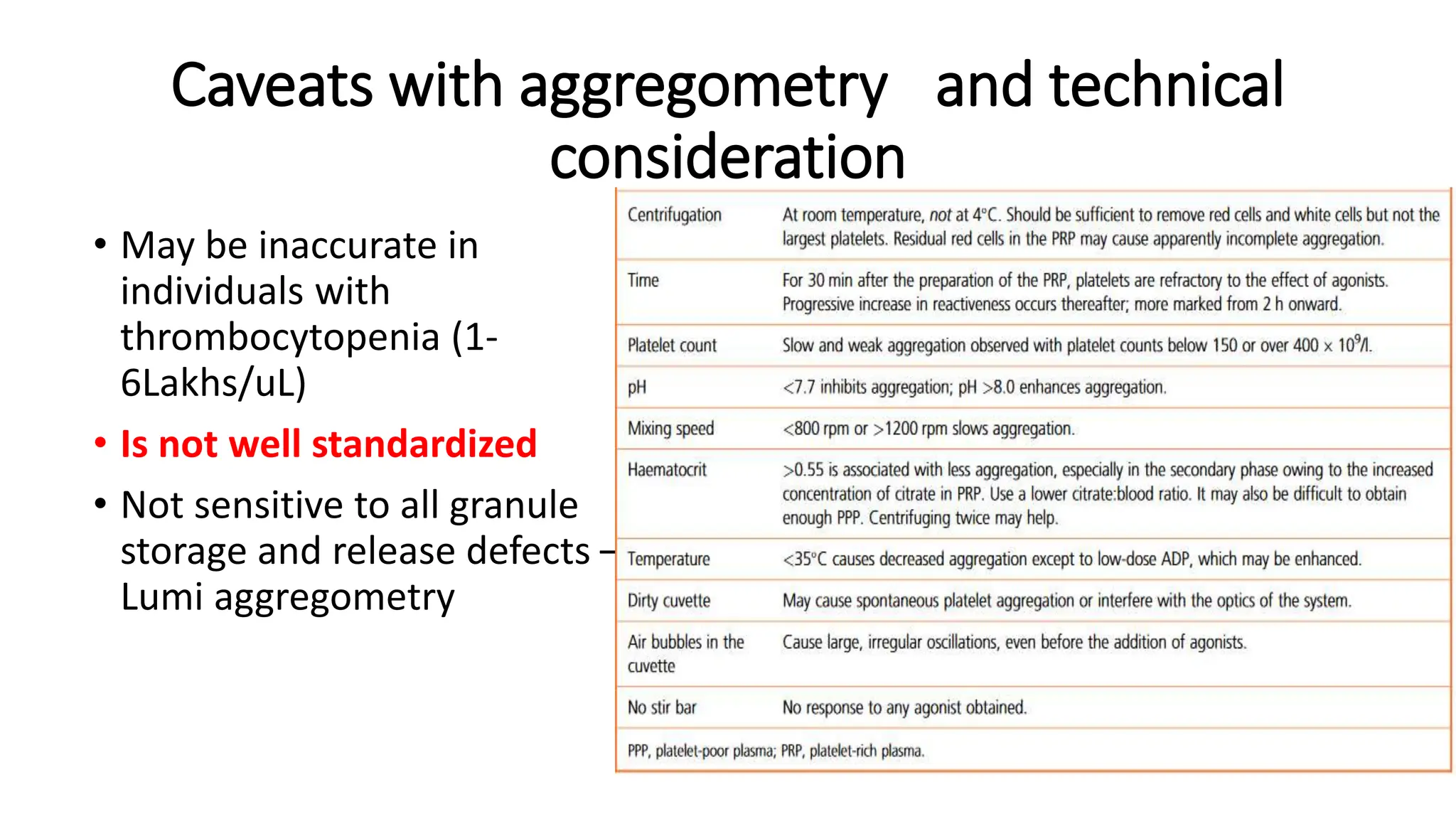
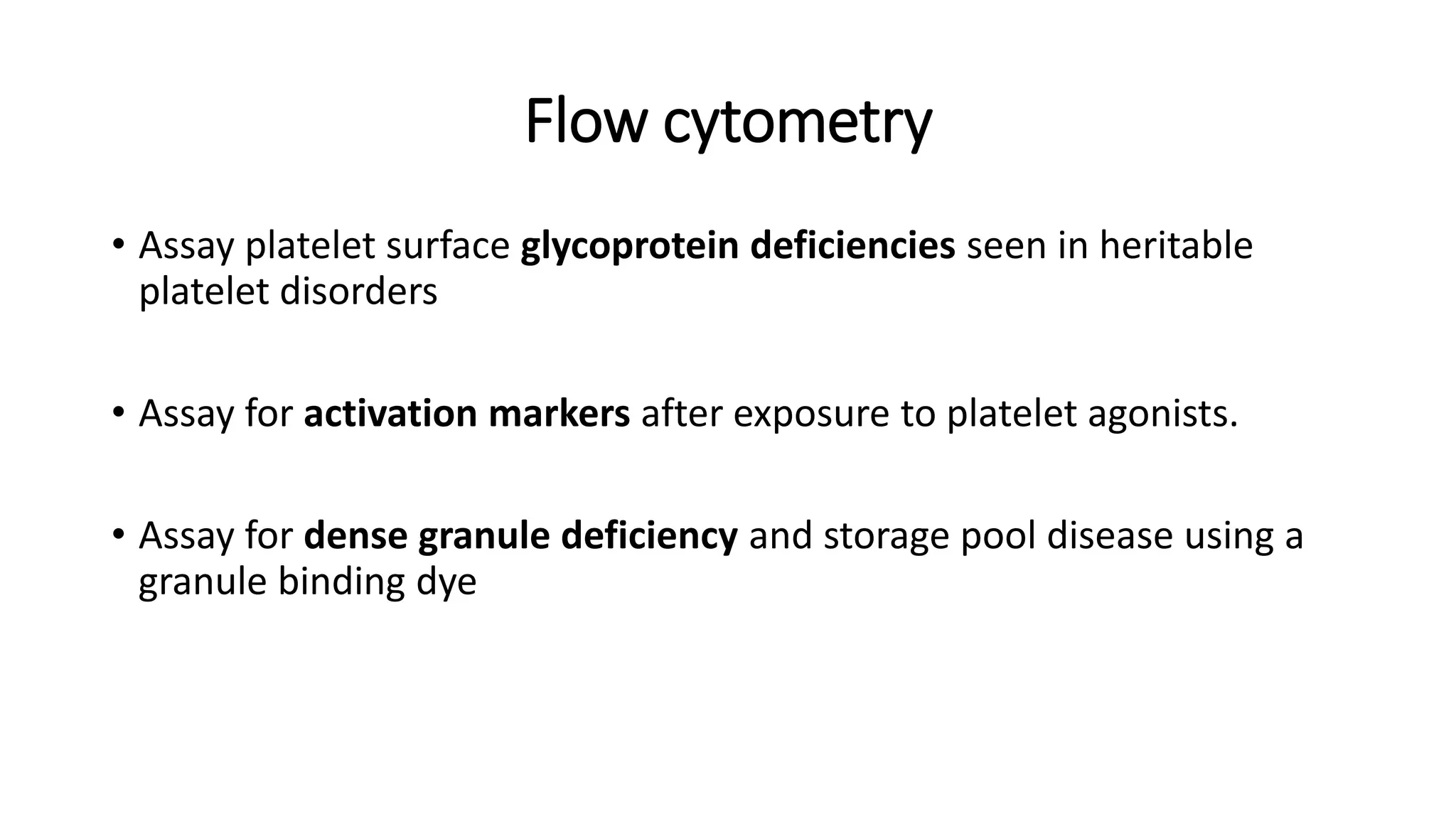
This document discusses platelet function disorders, assessment, and testing. It covers platelet ontogeny, normal platelet function, physiology, and important molecules involved in platelet function. Testing methods for assessing platelet function include platelet aggregometry, PFA-100, genetic testing, flow cytometry, and bleeding time. The document provides details on pre-testing considerations, sample collection and transportation, and interpretation of platelet aggregometry and other test results. It also discusses indications for platelet function testing and congenital platelet function disorders.