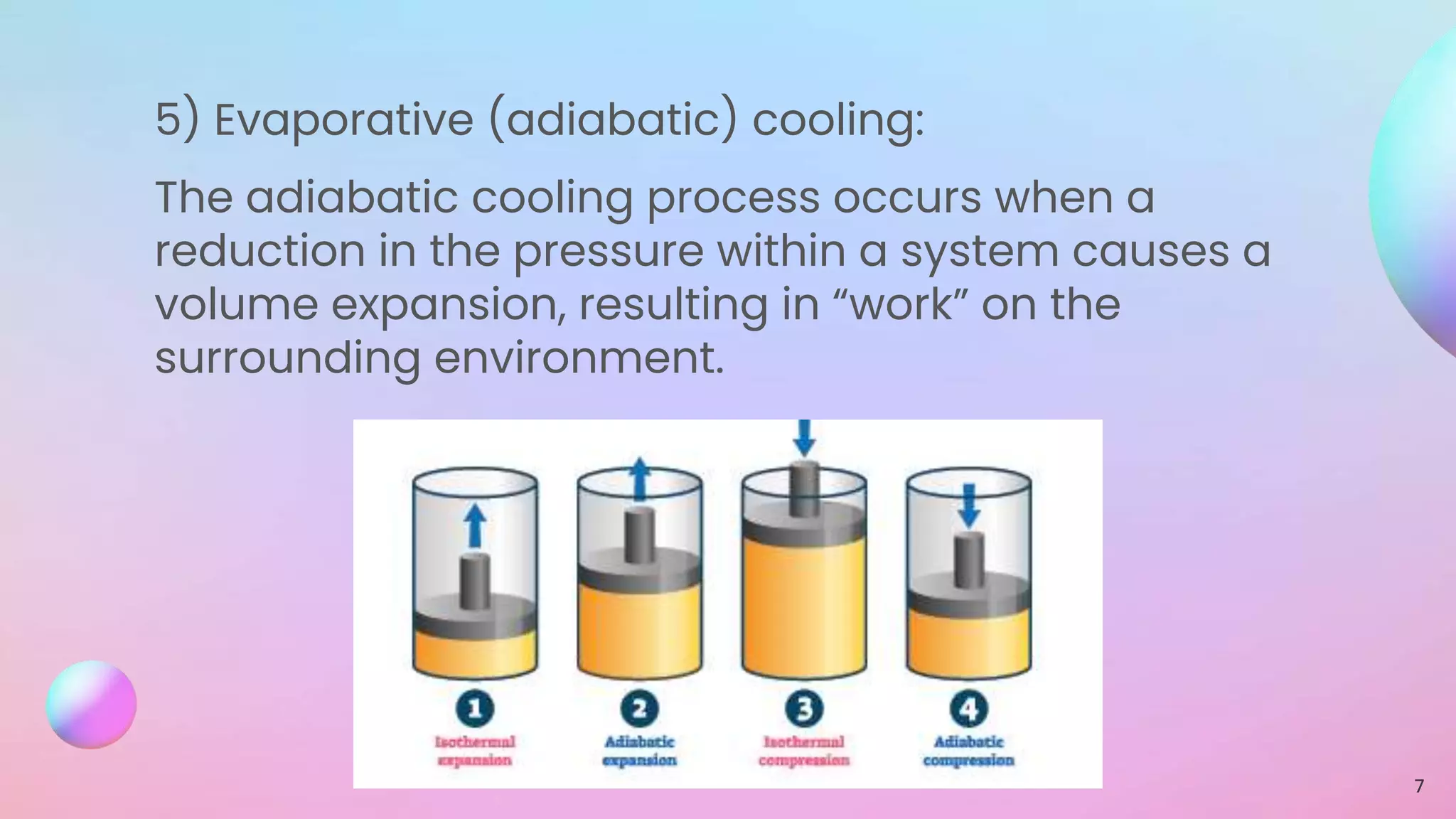
This document discusses various cooling processes in physics, including conduction, convection, radiation, perspiration, evaporative cooling, and laser cooling. It also covers gas behavior at low temperatures based on the kinetic molecular theory and Van der Waals forces, viscosity, superfluidity, and superconductivity.