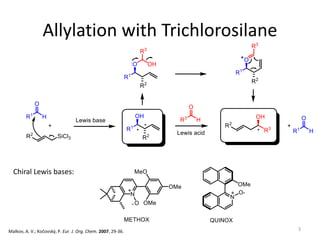
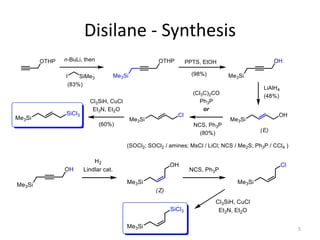
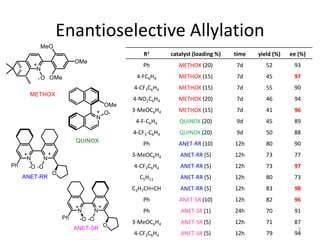
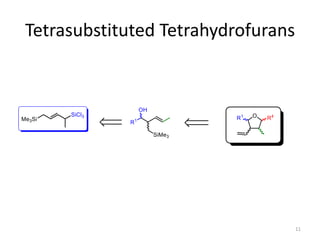
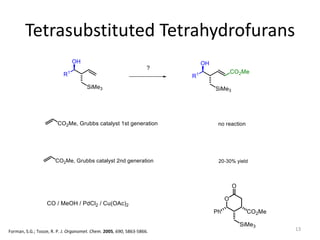
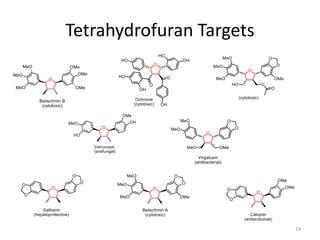
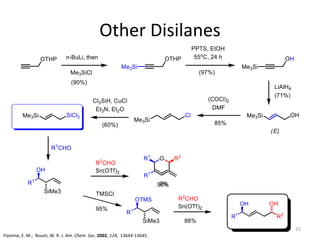
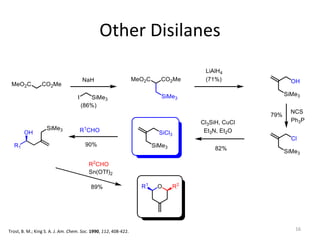
This document discusses the enantioselective synthesis of substituted tetrahydrofurans from homoallylic alcohols using chiral Lewis base catalysts and bifunctional disilane reagents. Specifically, it summarizes previous work using trichlorosilane and disilanes for allylation reactions. It then reports on the author's work exploring different catalysts for enantioselective allylation, achieving up to 97% enantiomeric excess. Various substituted tetrahydrofuran products are shown. The document concludes by discussing future directions for synthesizing tetrasubstituted tetrahydrofurans and exploring other disilane reagents.