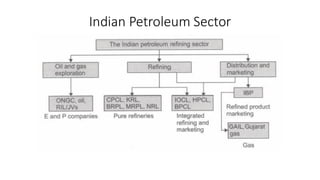
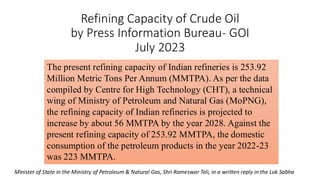
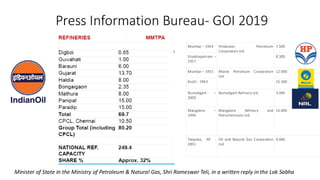
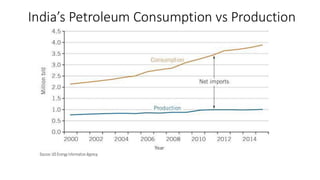
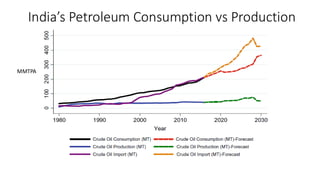
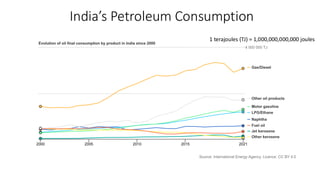
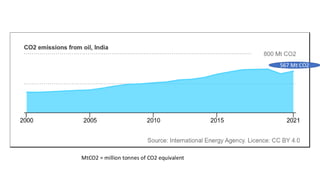
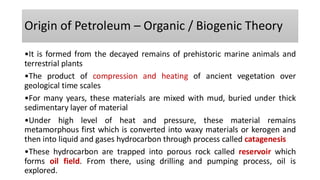
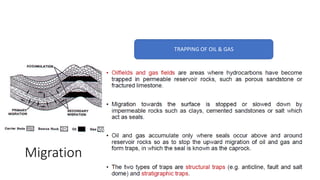
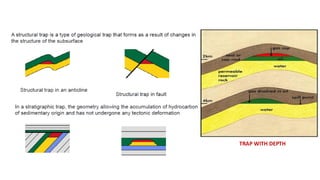
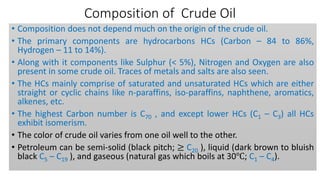
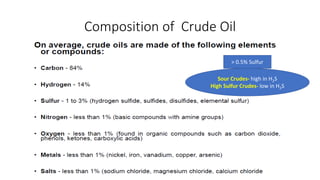
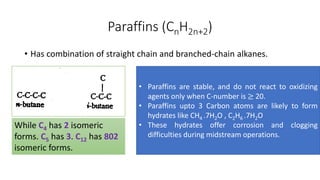
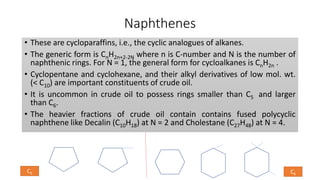
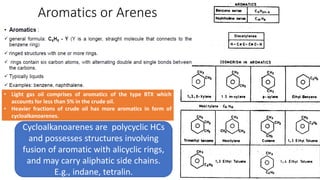
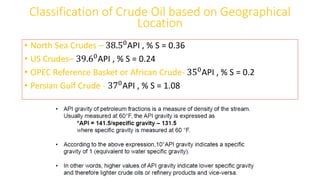
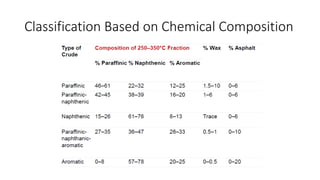
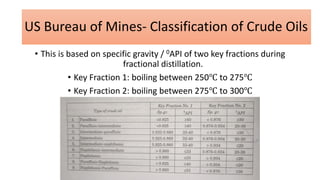
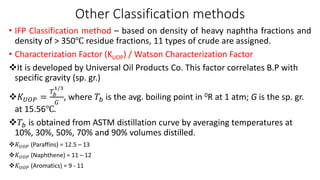
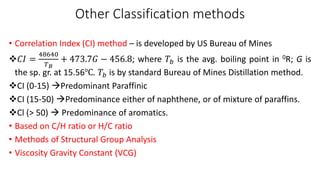
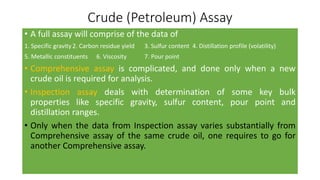
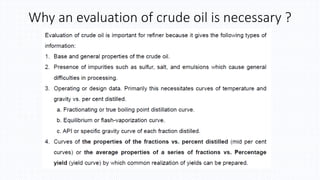
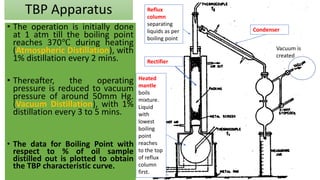
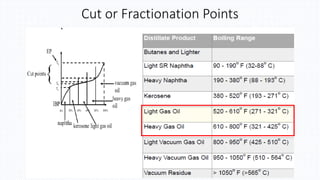
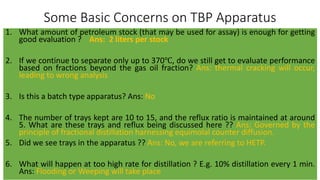
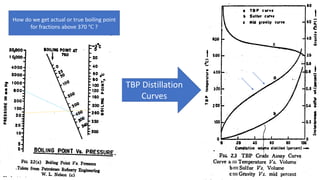
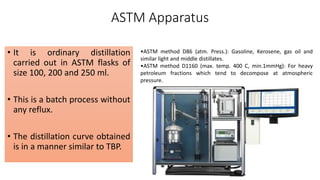
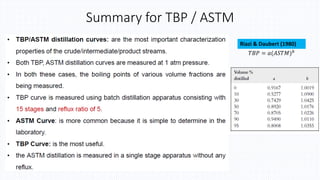
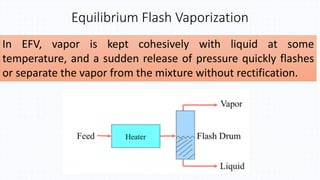
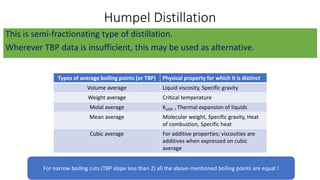
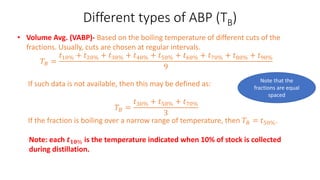
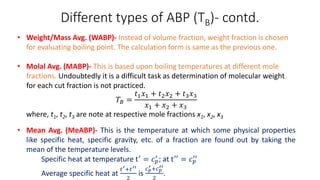
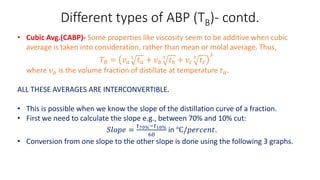
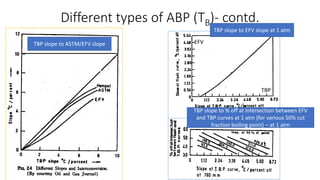
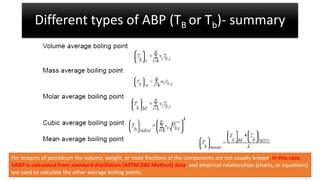
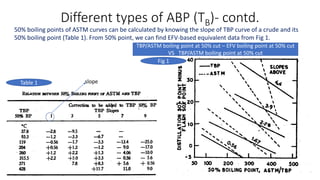
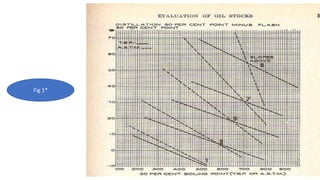
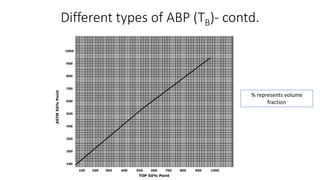
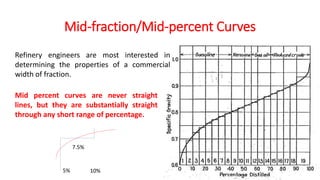
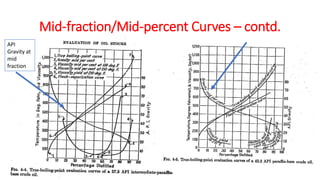
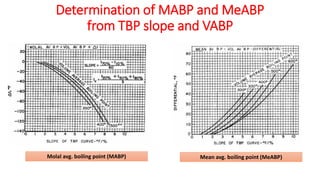
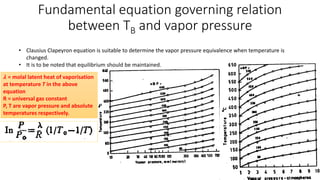
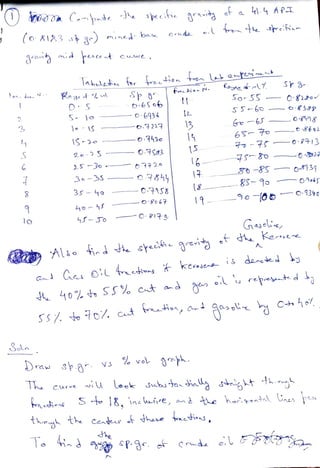
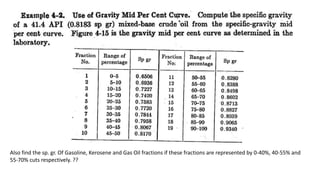
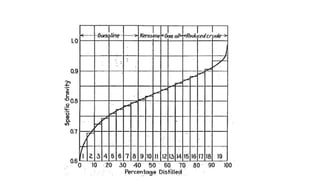
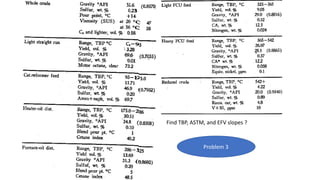
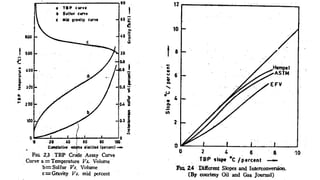
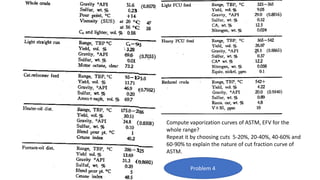
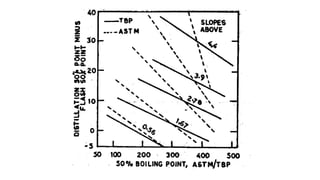
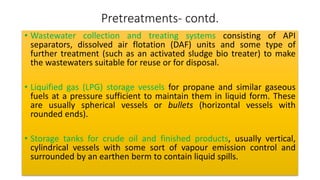
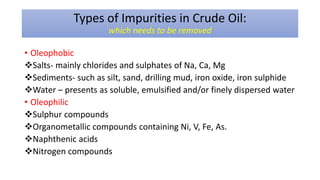
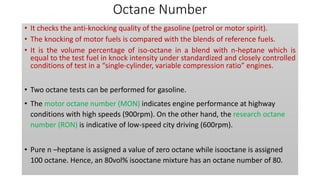
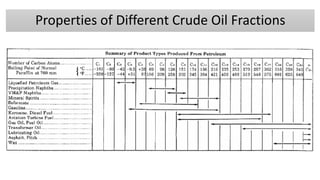
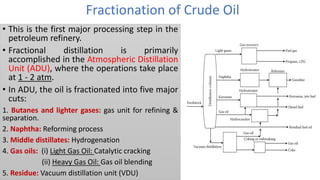
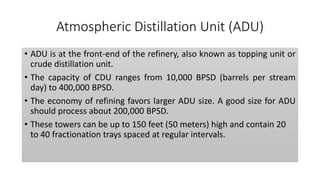
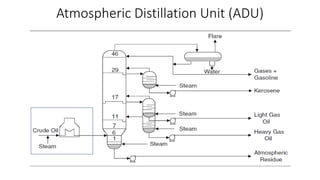
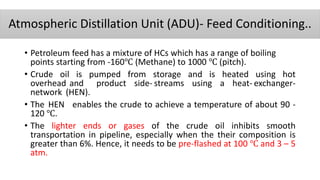
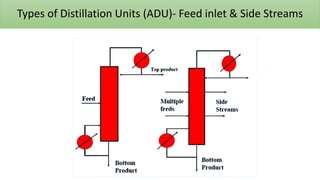
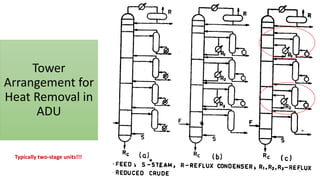
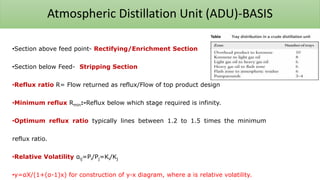
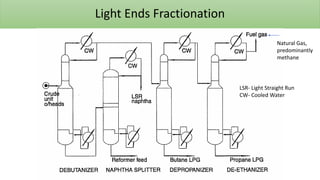
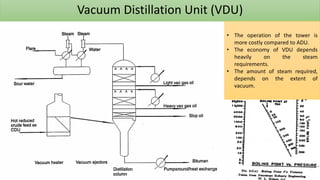
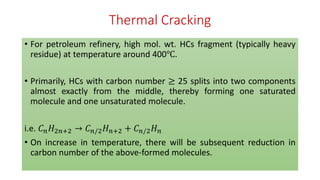
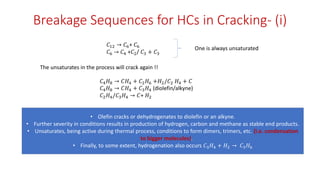
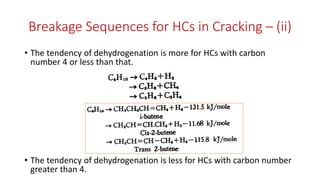
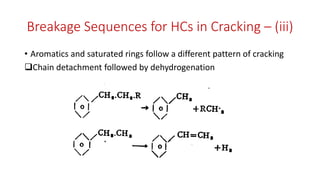
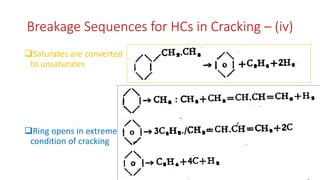
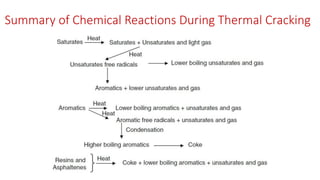
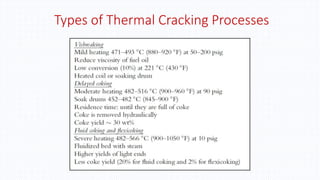
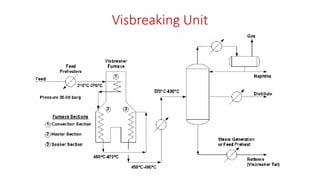
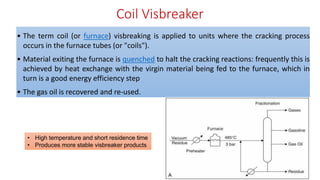
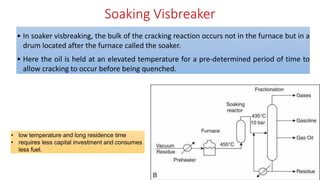
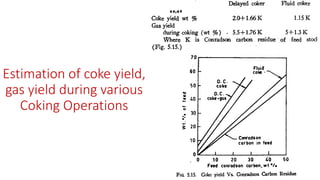
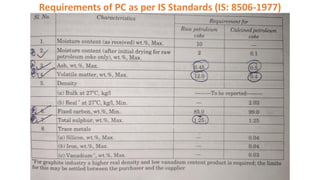
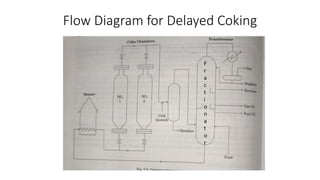
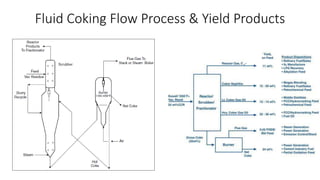
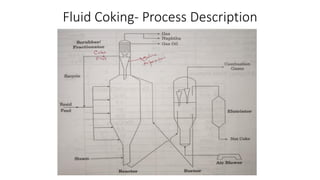
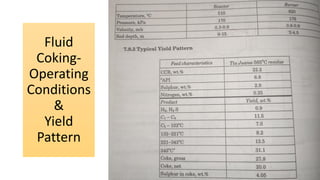
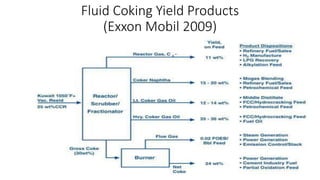
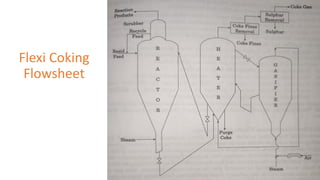
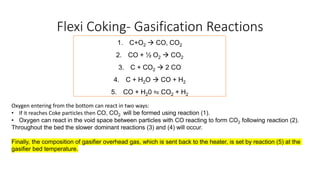
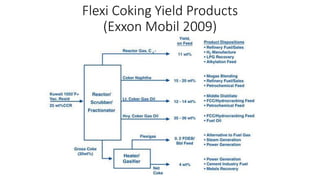
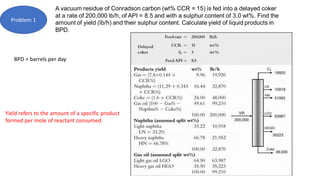
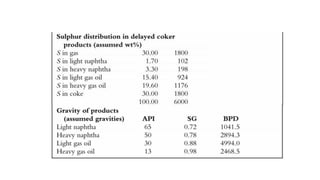
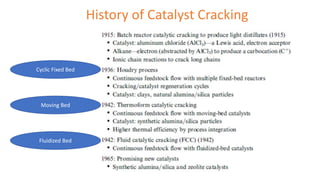
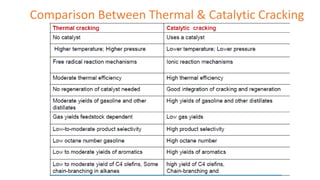
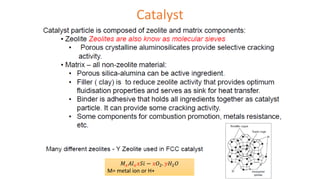
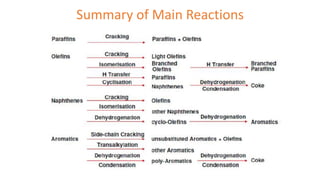
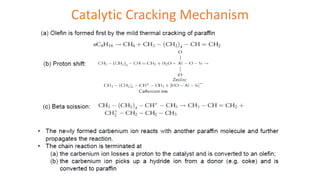
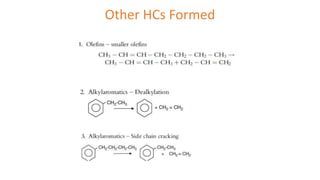
The document discusses petroleum refining operations and provides a historical overview of the oil and gas industry, including key milestones and the global reserves of petroleum. It highlights the current status of oil production and consumption in India, detailing the major refineries, their capacities, and the challenges faced. Additionally, it covers the composition of crude oil and its classification based on various factors, including geographical location and chemical properties.