Embed presentation
Download to read offline
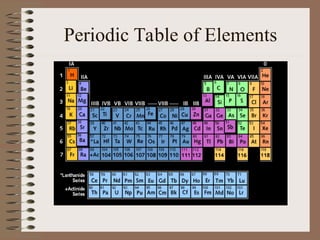
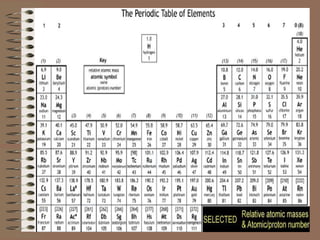
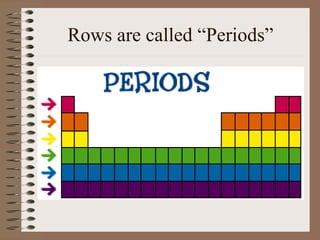
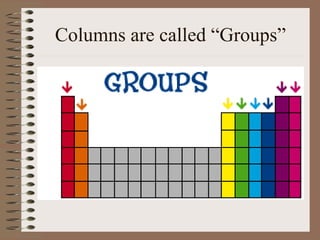
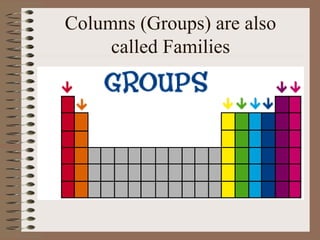
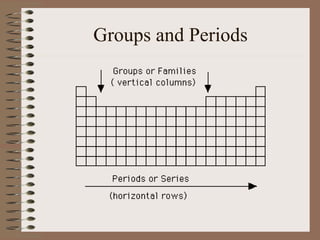
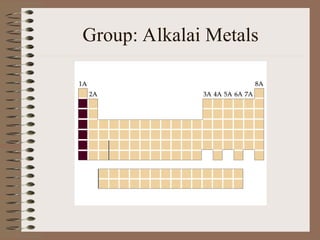
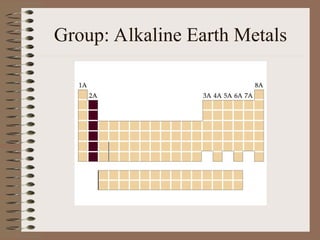
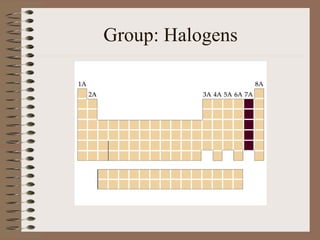
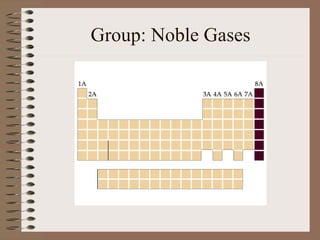
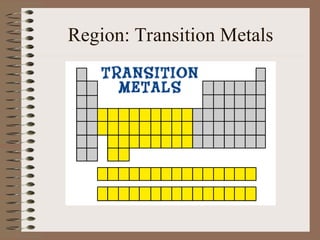
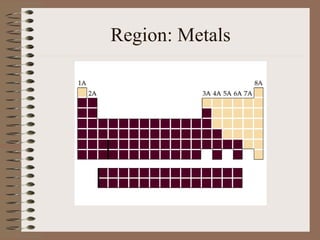
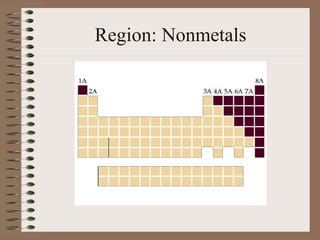
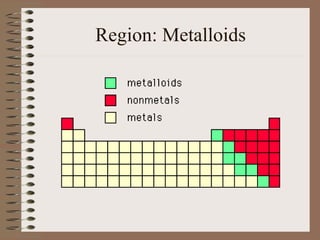
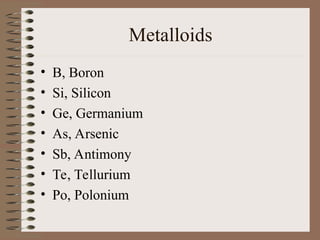
The periodic table organizes elements by increasing atomic number and highlights the periodicity of their physical and chemical properties related to atomic structure. Elements are arranged into rows called 'periods' and columns known as 'groups' or 'families', with specific groups including alkali metals, alkaline earth metals, halogens, and noble gases. Additionally, the table identifies regions for transition metals, metals, nonmetals, and metalloids.
















