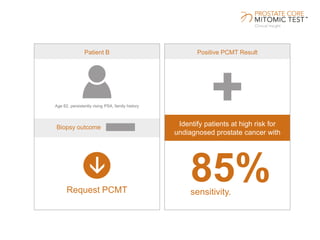
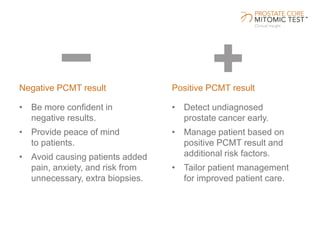
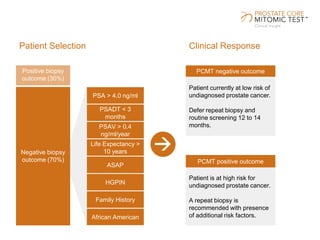
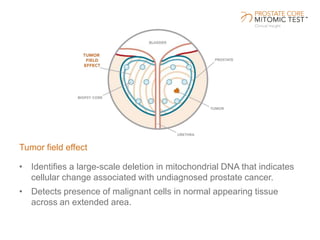
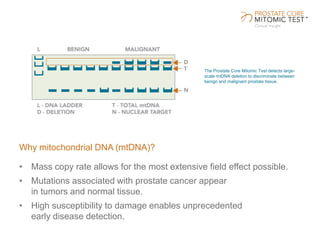
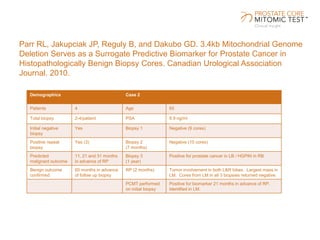
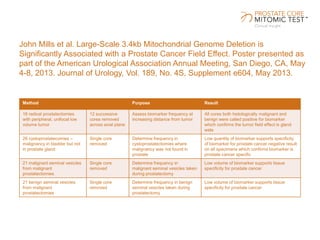
The document discusses a prostate cancer test called the Prostate Core Mitomic Test (PCMT) that detects large-scale mitochondrial DNA deletions to help determine if a negative prostate biopsy result represents a true negative or false negative. It provides examples of two patients (Patient A and B) where PCMT identified one as low risk for undiagnosed cancer after a negative biopsy (true negative) while identifying the other as high risk (false negative). The document promotes PCMT as helping physicians confidently stratify patients, avoid unnecessary repeat biopsies, and better manage patient care through early cancer detection.