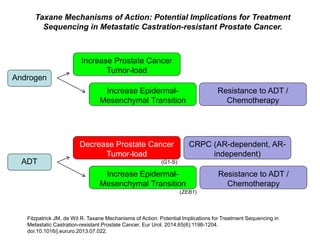
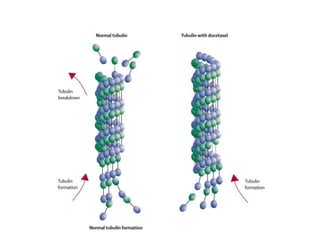
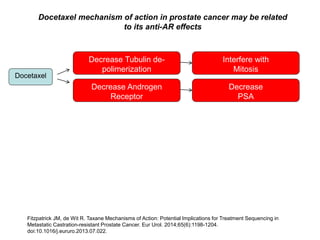
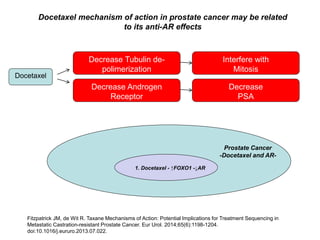
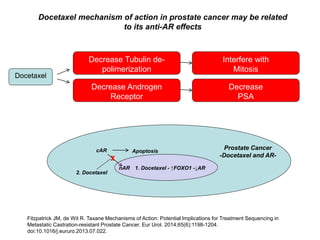
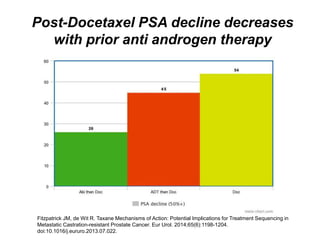
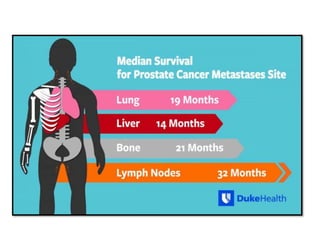
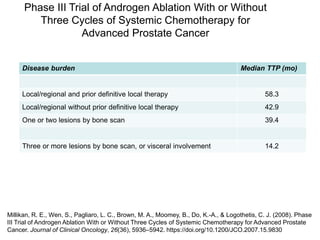
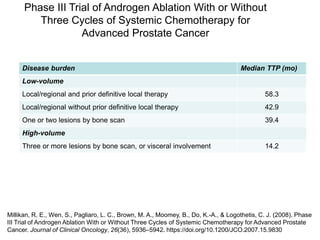
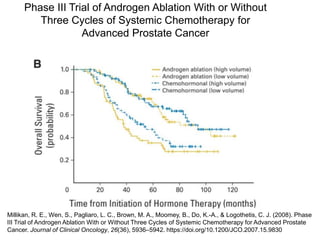
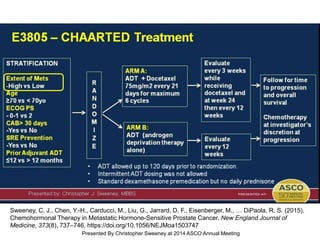
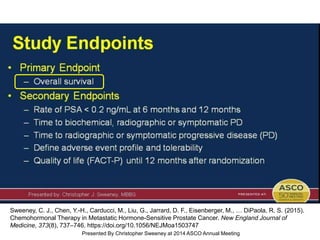
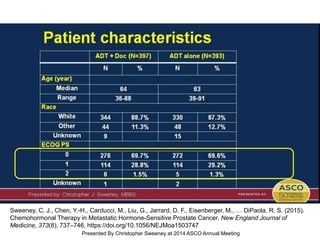
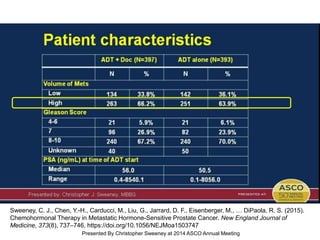
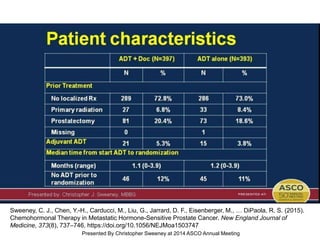
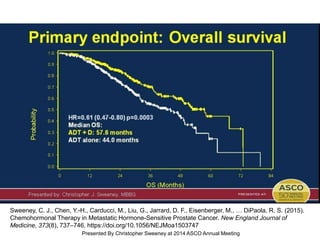
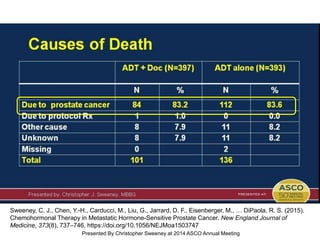
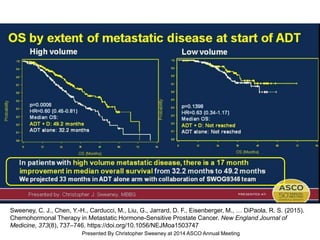
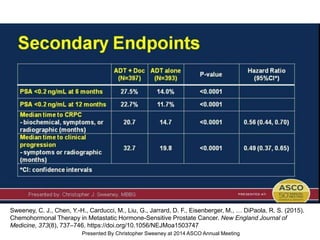
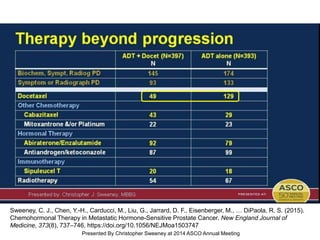
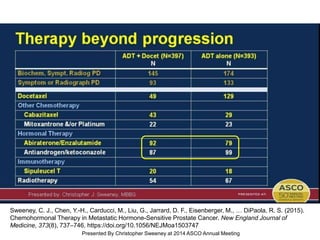
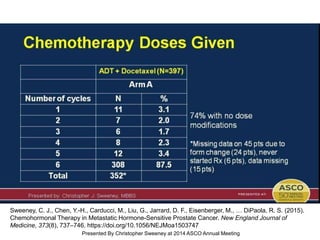
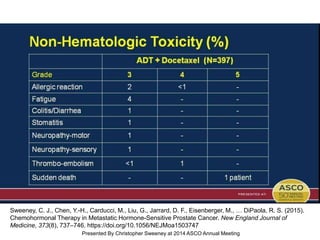
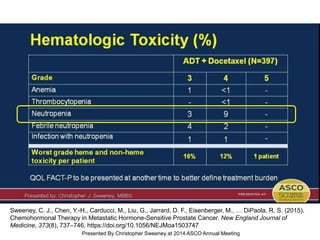
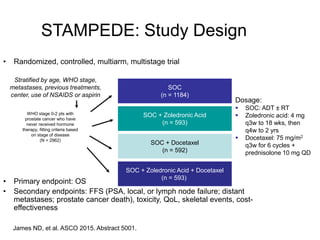
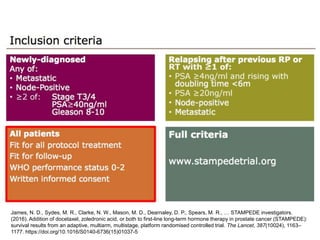
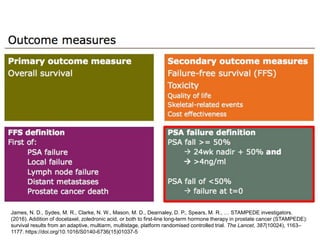
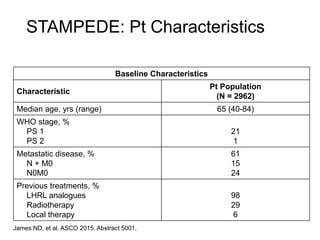
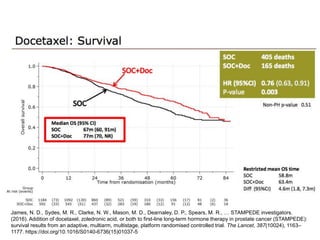
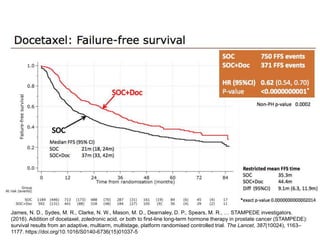
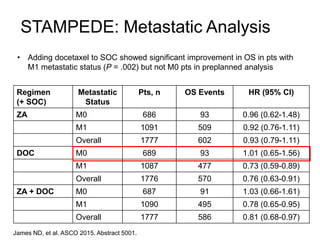
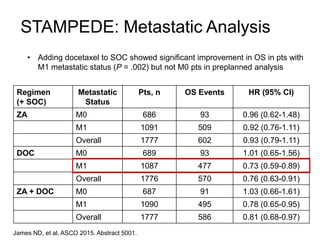
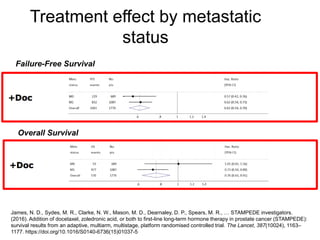
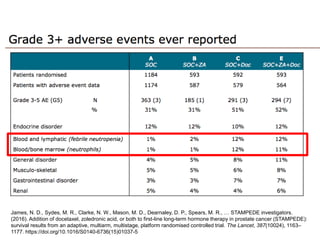
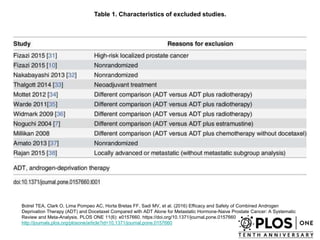
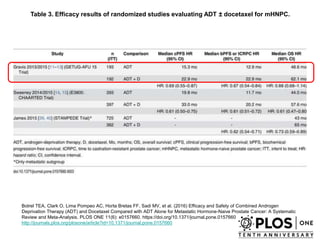
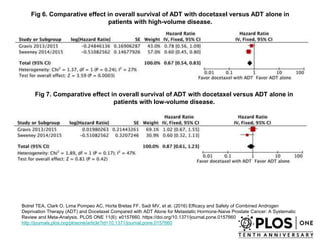
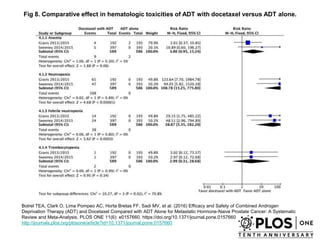
The document discusses chemohormonal therapy for castrate-sensitive prostate cancer (CSPC) and its potential benefits over standard androgen ablation. It highlights findings from a phase III trial that shows improved overall survival with the addition of docetaxel to androgen deprivation therapy. The document also mentions key statistics and results presented at the 2014 ASCO annual meeting, emphasizing the effectiveness of this treatment approach for advanced prostate cancer.