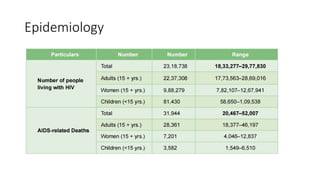
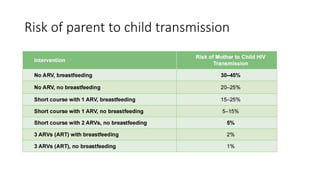
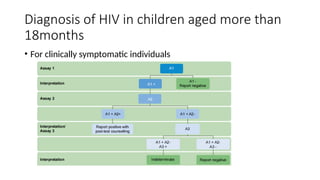
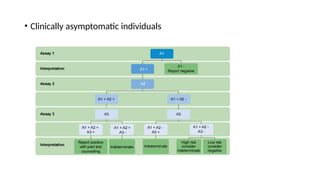
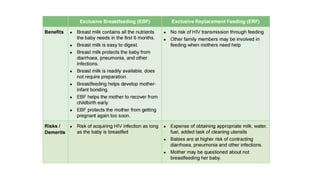
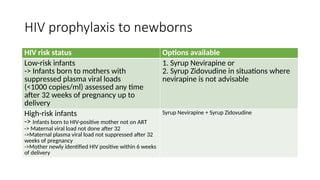
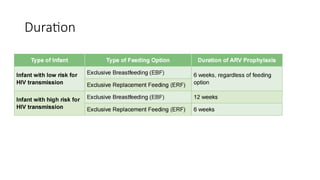
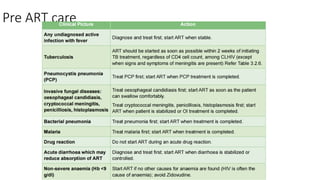
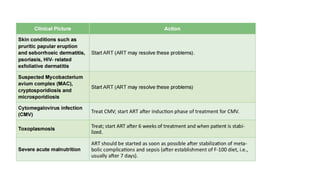
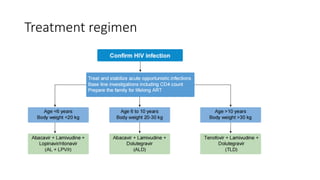
The document outlines pediatric HIV treatment guidelines, emphasizing the diagnosis and management of HIV in children under 18 months through laboratory tests and maternal antibody considerations. It provides feeding recommendations for HIV-exposed infants, including breastfeeding with ARV interventions and guidelines for complementary feeding, as well as immunization and treatment regimens. It also details the mechanisms of various drug classes used in ART, highlighting the importance of monitoring and addressing treatment failures in children.