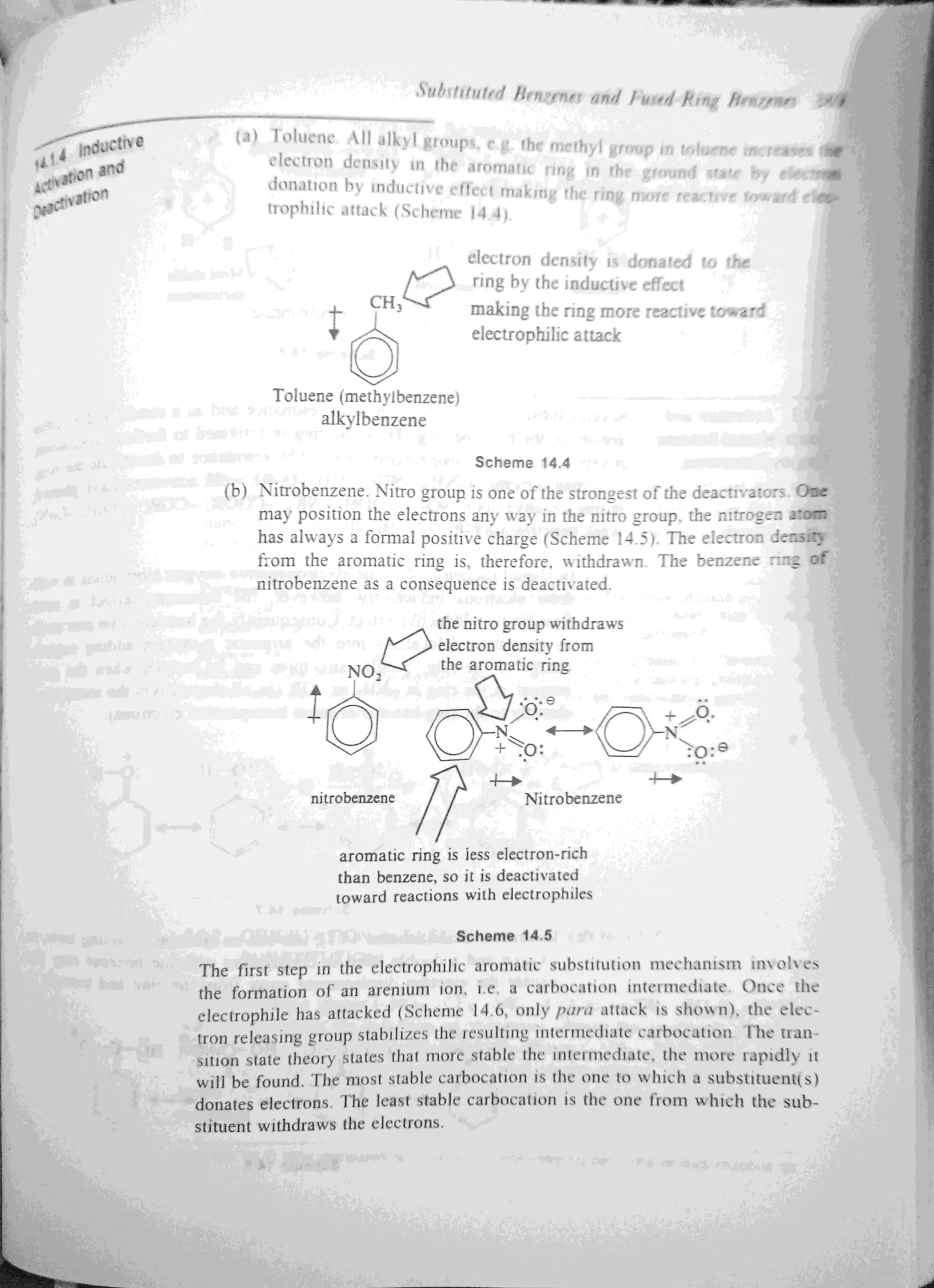
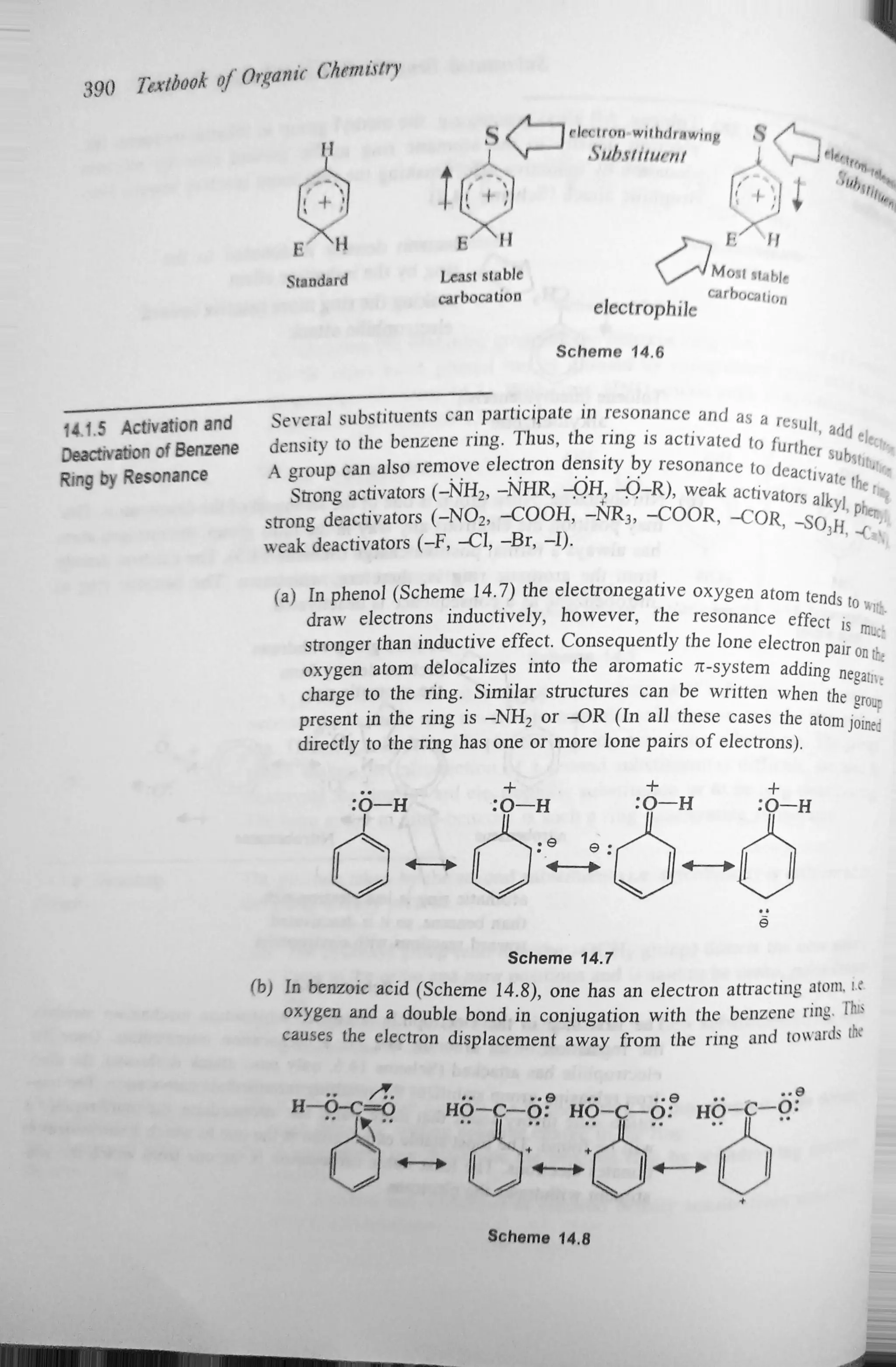
The document discusses the activation and deactivation of the benzene ring through various substituents and their effects on electrophilic aromatic substitution. It explains how electron-donating groups can activate the ring, while electron-withdrawing groups can deactivate it, influencing the positions of new substituents. Additionally, it outlines the role of resonance and inductive effects in determining the stability of carbocations and the overall reactivity of the aromatic compounds.

![Ond Ripe
group. Conseqtrmntly
the ieqonance
the ring. resonance (an be wr'ften for
(Scheme14.9),
-o o O o o
Scheme 14.9
In summary, the relative order of carbocationstability and the relative elec-
tron concentration on the aromatic ring are the two ways to guide the activation
and deactivation of the ring. The groups which donate or release electron
density to the aromatic ring activate it for further substitution. On the other
hand, the groups which withdraw electrons from the aromatic ring deactivate It
for further substitution.
Problem 14.1: Alkylgroups are activatingsubstituents. The initialproductof Friedel-
Crafts alkylation is much more reactive than the starting material, thus multiple
alkylationsmay be difficultto avoid. How can one solve this problem?
Answer 14.1: By carrying out a Friedel-Craftsacylationfollowedby Clemmensen
reduction of the ketone. The acyl benzene formed initiallyhas a carbonyl group bonded
to the aromatic ring (Scheme 14.10), i.e. RCO- is an deactivating group. Once
introduced it deactivates the ring for further substitution.
o
R—c—a
AJC13
acylbenzene
Scheme 14.10
(a deactivating group)
14.2 ORIENTATIONIN ELECTROPHILICAROMATIC
SUBSTITUTION
Thedeactivatinggroups (-N02, -CF3, -NR3,-COOH, -COR, .S03H, -CEN)
whether operating by induction or resonance,direct the incoming electro-
philes to the meta position, while activatinggroups (—NH2,
--NHR,NR., —OH,
—OR,
alkyl, phenyl) direct to theortho and para positions. Halogen substi-
tuents(—Br,
-C], —I,F) although deactivating,direct ortho and para are an
exception.
Problem 14.2: Why compared to toluene,(trifluoromethyl)
benzene is less reactive to](https://image.slidesharecdn.com/orientation-250205125525-2026a56a/75/orientation-and-reactivity-of-benzene-substituitents-4-2048.jpg)