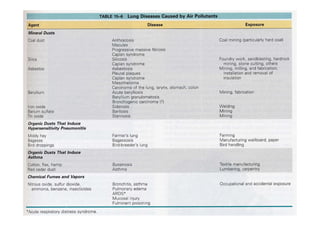
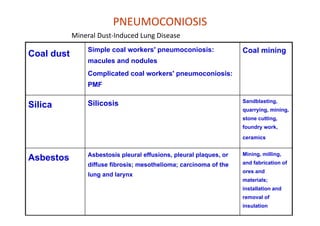
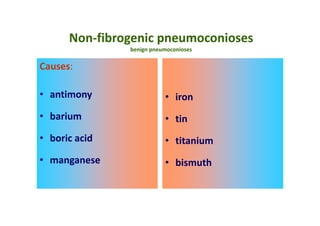
The document discusses various occupational lung diseases, including pneumoconiosis, hypersensitivity pneumonitis, silicosis, asbestosis, and organic dust-related conditions. It details the pathogenesis, clinical features, diagnosis, and management of these diseases, emphasizing factors such as dust exposure, particle characteristics, and the role of irritants like tobacco. Key examples include coal workers' pneumoconiosis, silicosis from silica dust, and asbestosis resulting from asbestos exposure, each with specific symptoms, complications, and treatment considerations.