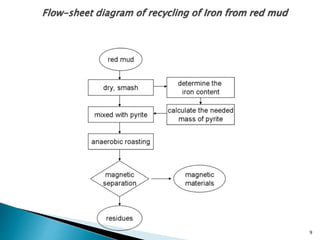
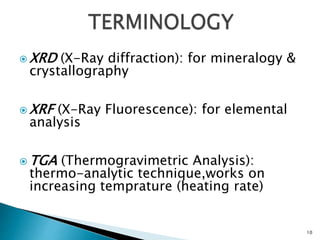
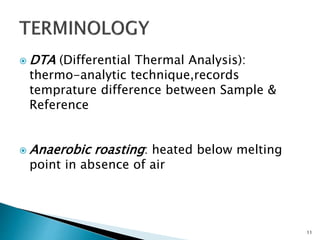
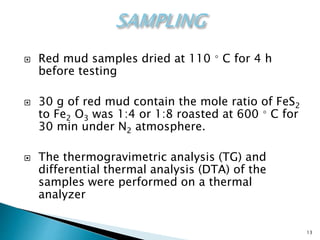
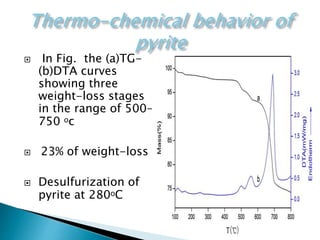
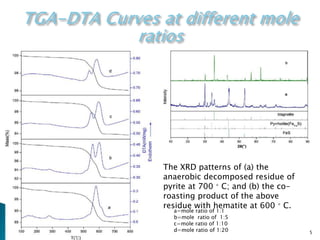
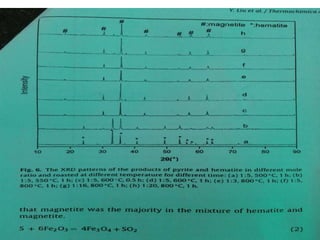
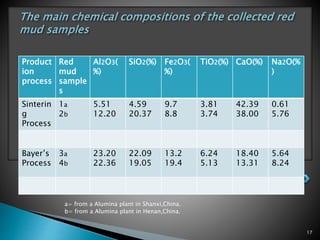
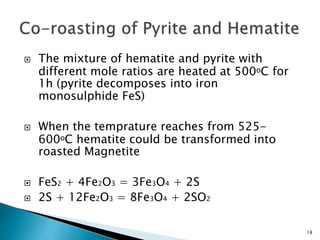
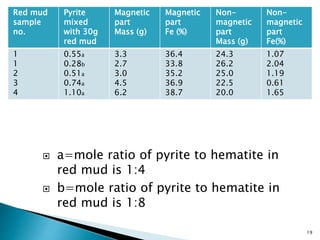
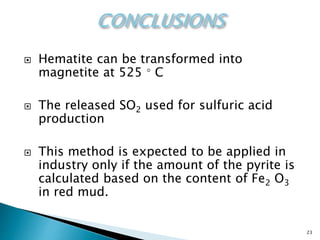
Red mud is a toxic residue from bauxite processing that contains 10-30% hematite. This document proposes a method to transform the hematite in red mud into magnetite, the highest grade iron ore, using pyrite. Laboratory experiments showed that mixing pyrite and red mud in a 1:4 or 1:8 ratio, then roasting under nitrogen at 600 degrees C, results in 23% weight loss as the pyrite decomposes and sulfur is released. X-ray diffraction analysis identified the product as magnetite. On a larger scale, 1 ton of red mud mixed with 40 kg pyrite can produce 232 kg of magnetite concentrate containing 38.7% iron. The released sulfur dioxide could also be used