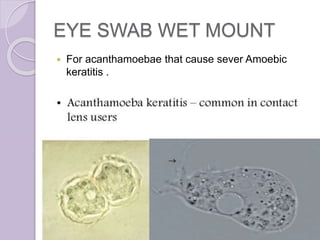
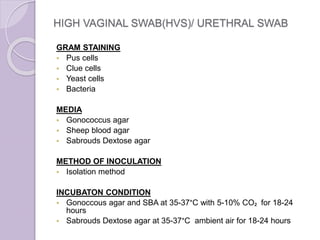
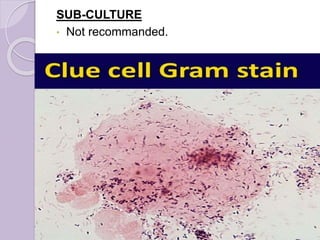
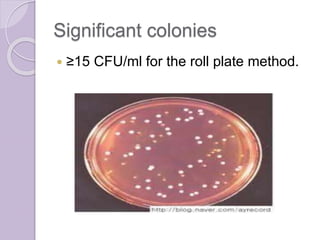
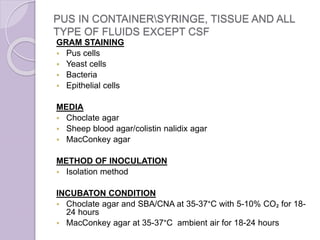
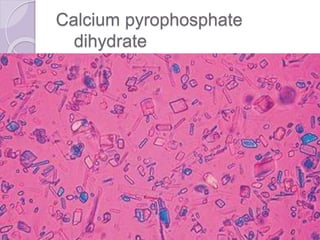
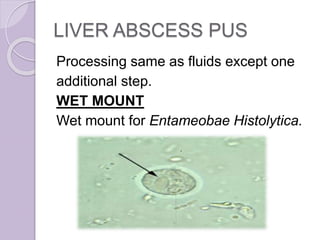
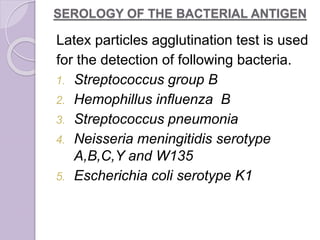
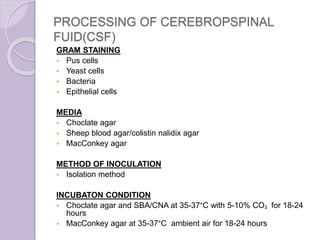
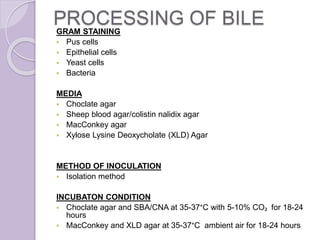
This document provides instructions for processing different clinical specimens for microbiological culture. It describes the recommended media, incubation conditions, gram staining procedures, and sub-culturing steps for specimens including swabs, blood, urine, stool, sputum, and cerebrospinal fluid. Precise methods are outlined for maximizing recovery of aerobic and anaerobic bacteria from each specimen type.