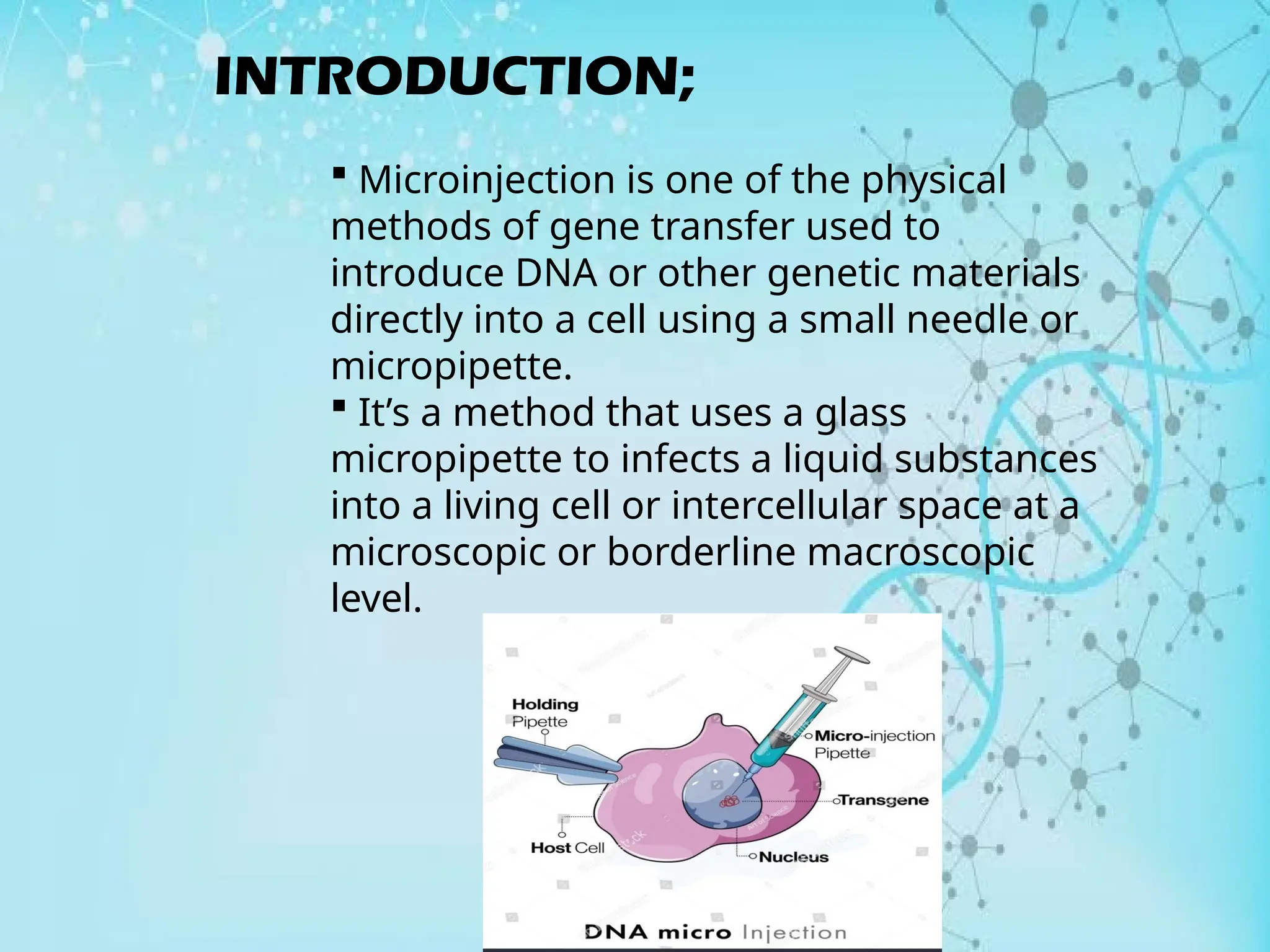
The document provides an overview of microinjection, a gene transfer technique using a micropipette to deliver DNA into cells. It discusses the history, principles, steps, applications, advantages, and disadvantages of microinjection. Key applications include the creation of transgenic animals and its use in in vitro fertilization and neuroscience research.