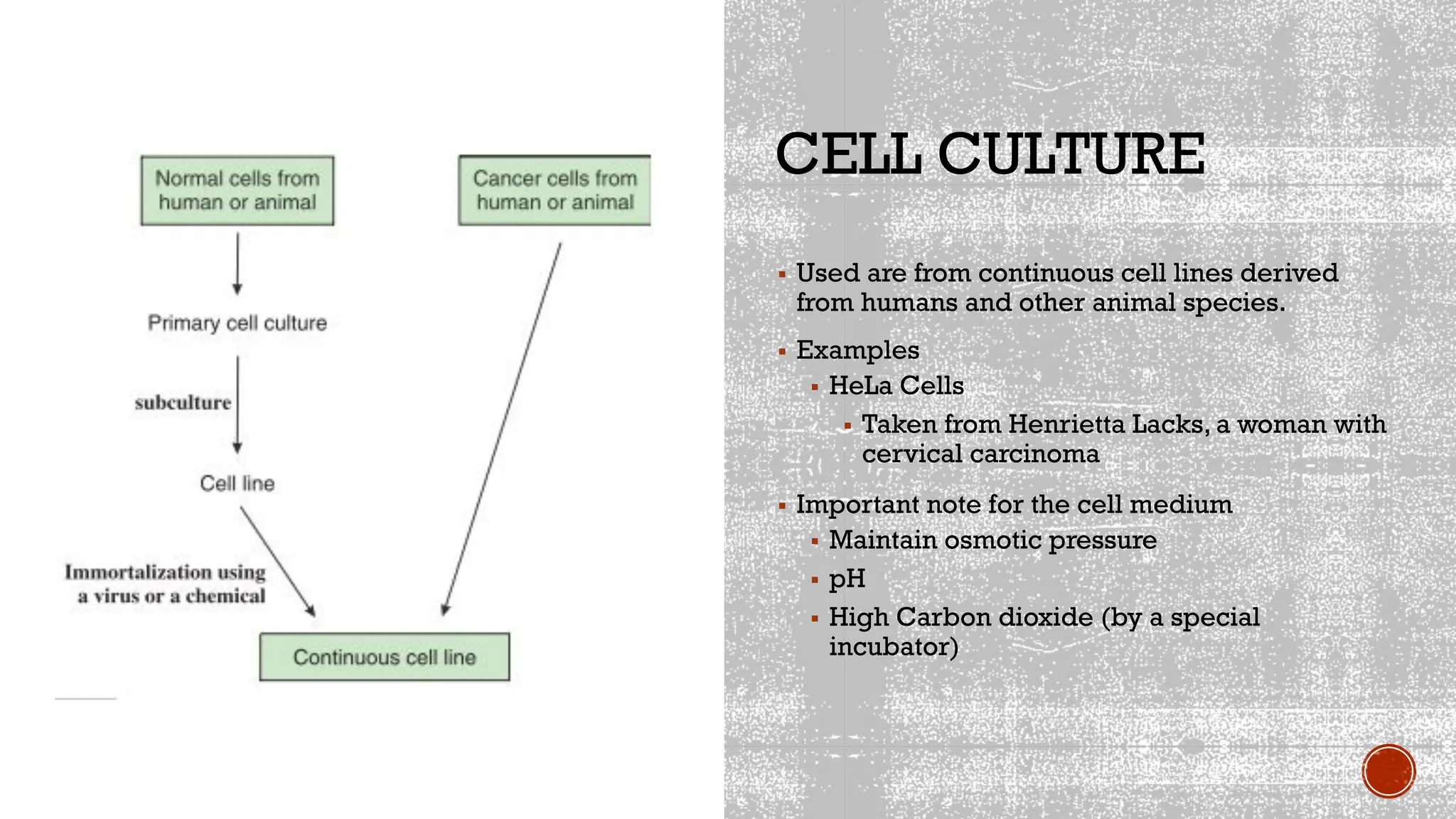
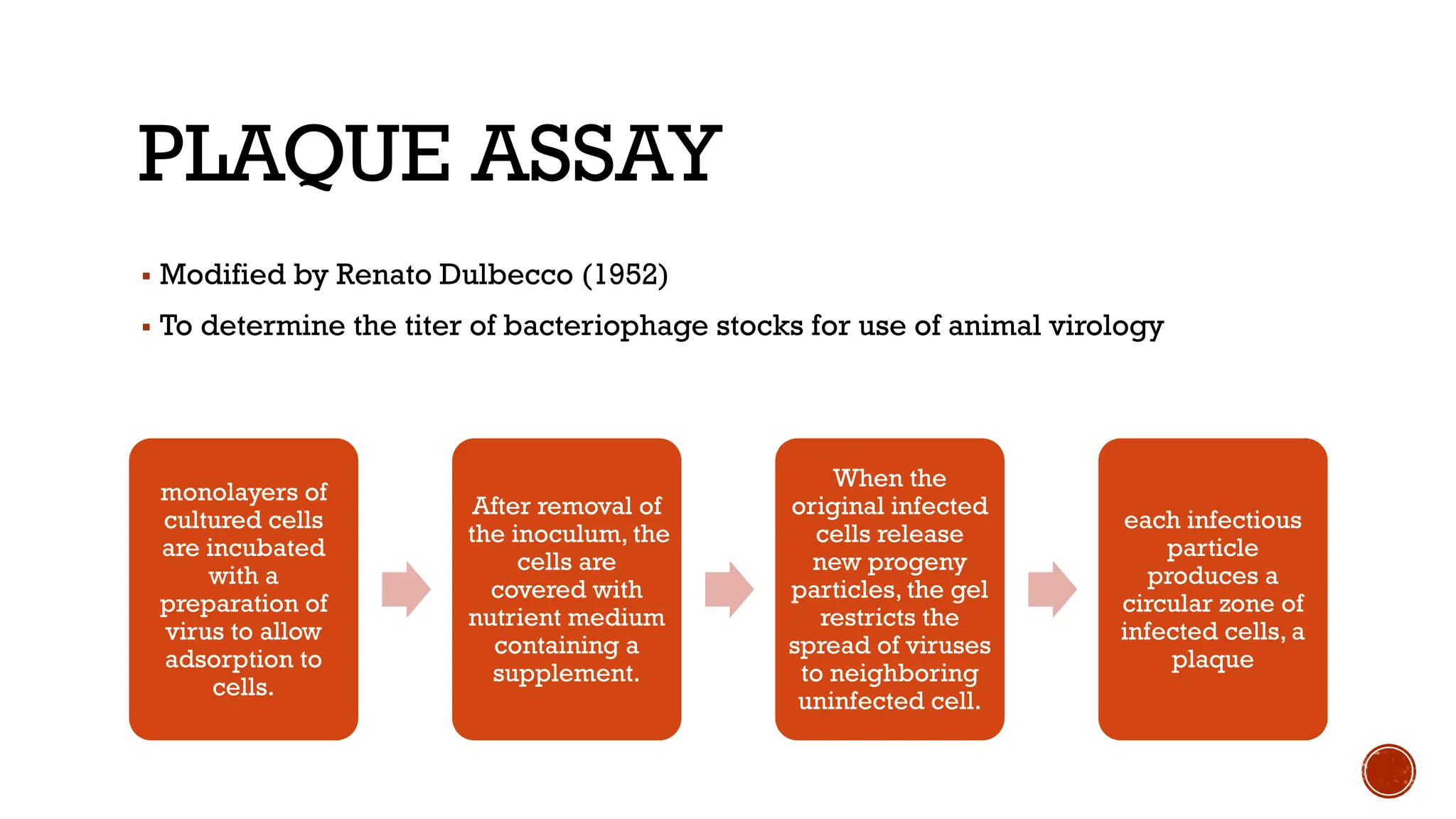
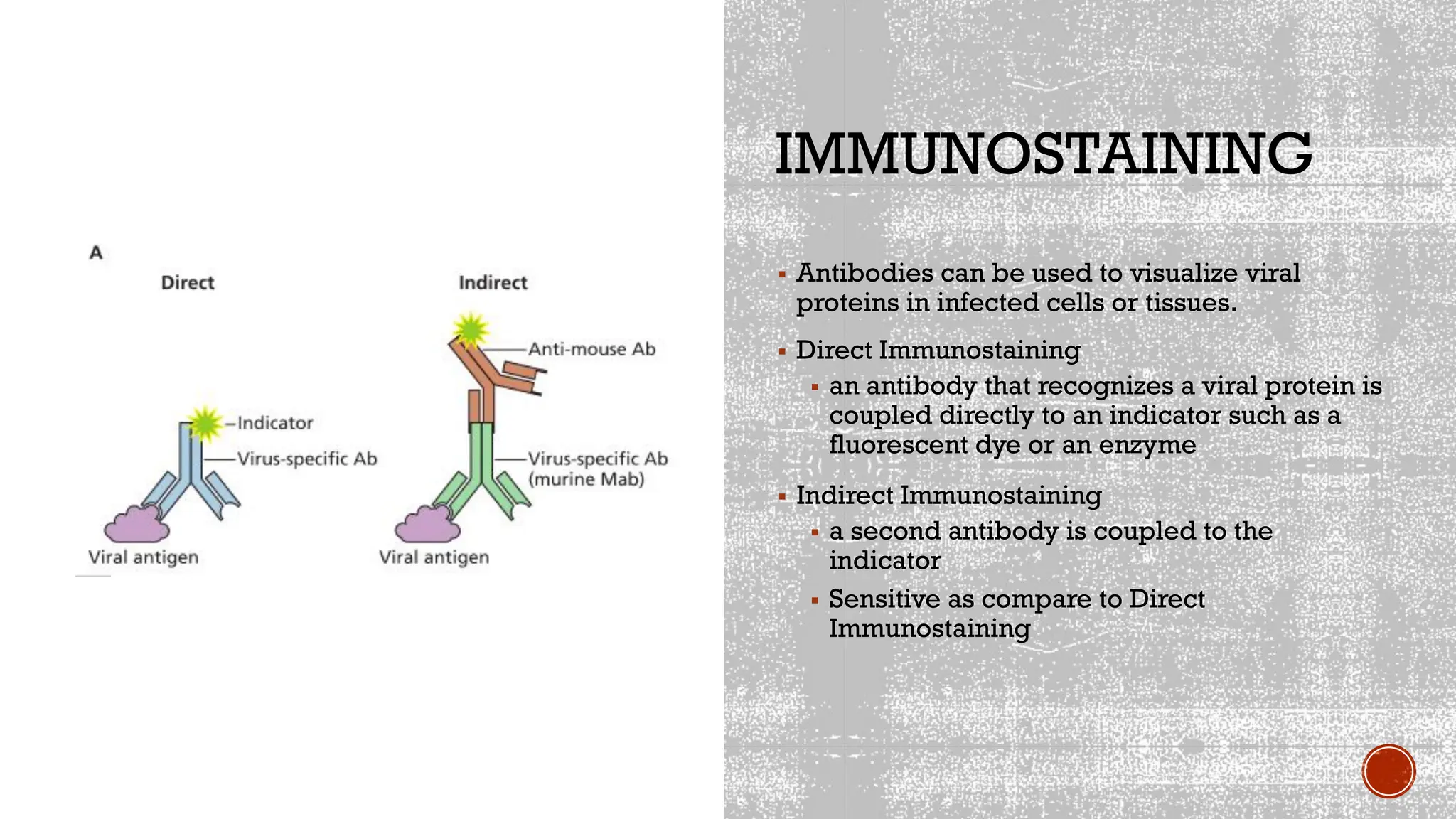
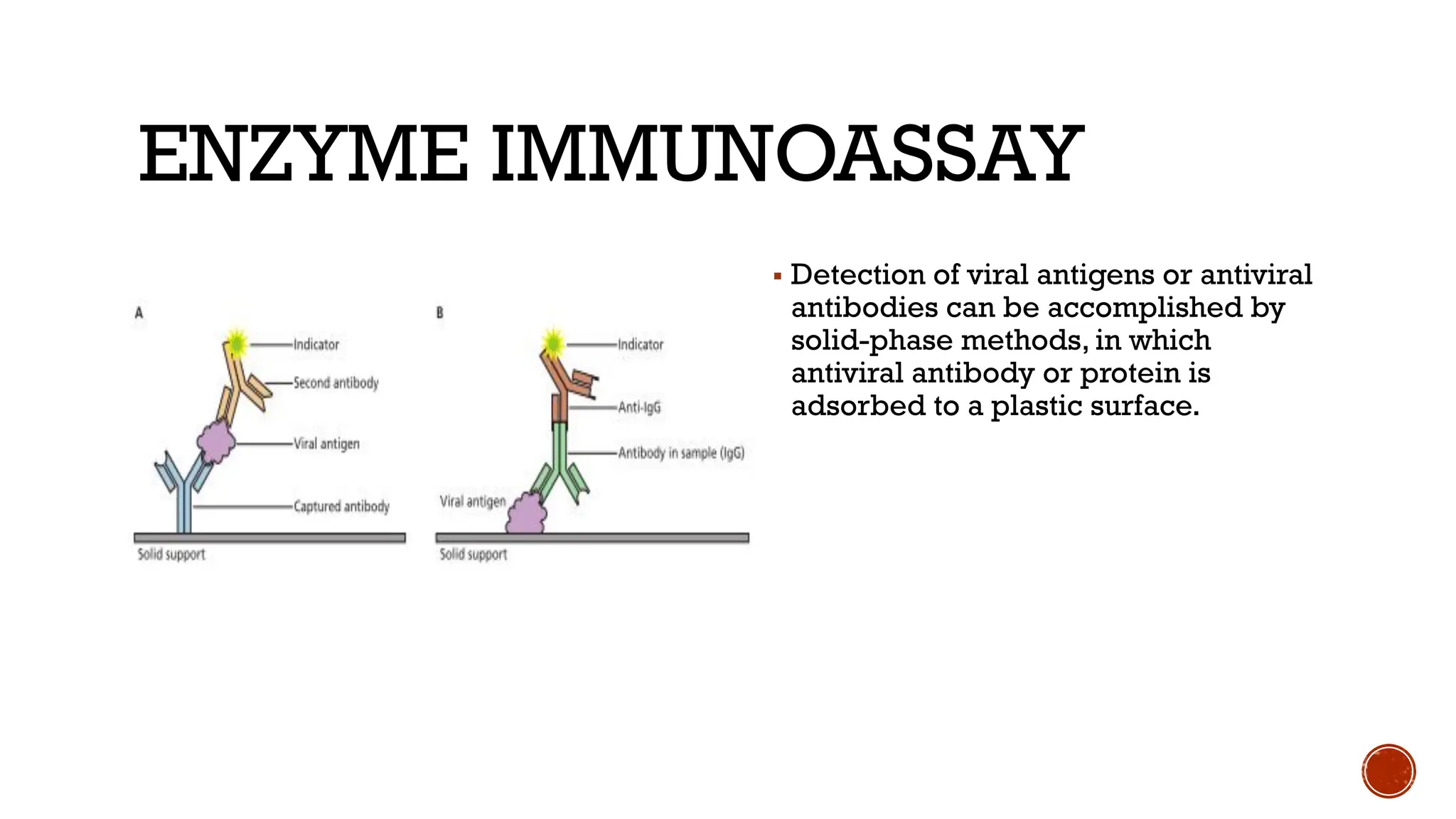
The document outlines various methods used in virology for virus identification, including cytopathic effects, serological tests, and viral nucleic acid detection techniques like PCR. It details virus isolation and culture techniques, emphasizing the use of continuous cell lines and embryonated eggs for vaccine production, as well as the assays employed to detect viruses, both biological and physical. Additionally, methods such as immunostaining and enzyme immunoassays are discussed for detecting viral proteins and nucleic acids.