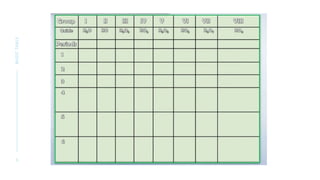
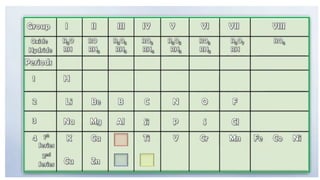
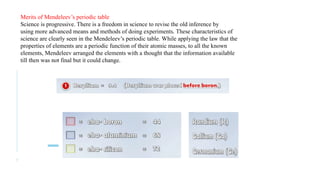
Dmitri Mendeleev developed the periodic table of elements by organizing 63 known elements based on their atomic masses and properties. His work was a significant advancement in the classification of elements, emphasizing that the properties of elements are periodic functions of their atomic masses. Mendeleev's periodic table exemplified the evolving nature of scientific understanding, allowing for revisions based on new discoveries.