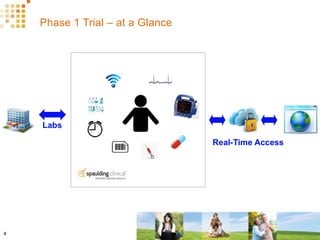
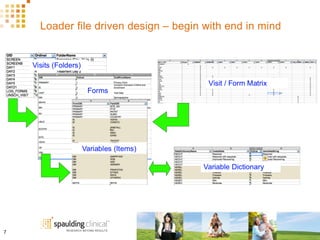
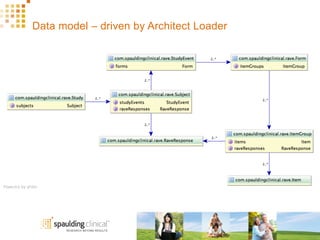
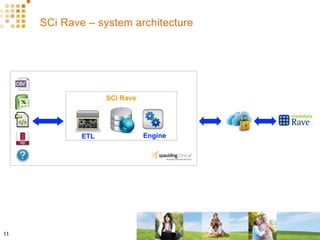
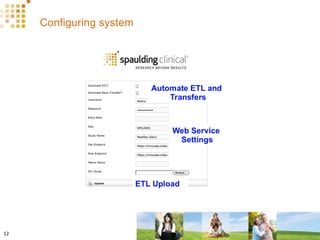
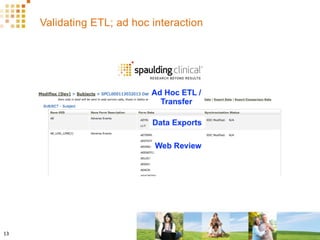
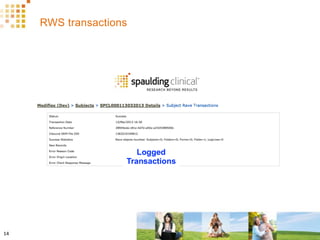
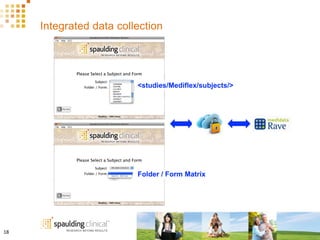
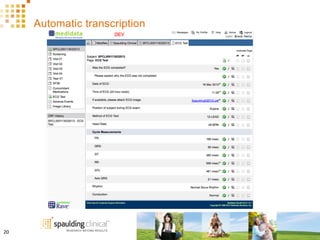
The document discusses how Spaulding Clinical leverages Rave Web Services (RWS) to integrate their phase 1 clinical trials seamlessly with sponsors' Rave electronic data capture (EDC) systems. It describes Spaulding's SCi Rave system, which uses a loader file, extract-transform-load processes, and an interface engine to transfer study data from Spaulding to Rave in near real-time or on scheduled transfers. The system architecture is flexible and has successfully transferred thousands of records across multiple studies with no manual data entry needed. The document concludes by envisioning further integration of devices, sensors and other data sources using systems like RWS.