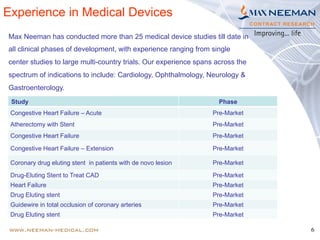
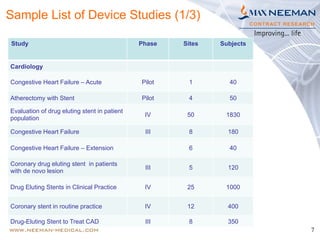
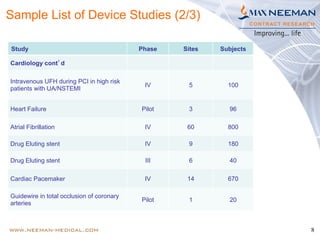
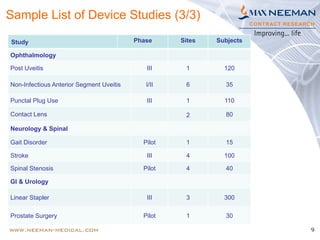
Dr. Jayashri Krishnan is a seasoned clinical research professional with over 17 years of experience in medical devices, specializing in dermatology and ophthalmology. The document outlines the growth and regulatory landscape of India's medical device market, which has seen significant expansion, and highlights Max Neeman's extensive experience in conducting clinical trials for various medical devices across multiple therapeutic areas. Additionally, it presents several case studies demonstrating Max Neeman's successful execution of clinical trials in cardiology, urology, and gastrointestinal procedures.